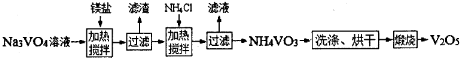
��Ŀ����
���Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ��ά���ء���Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϣ����ֺ���������ˮ�е��ܽ������±���ʾ��
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |

��ش��������⣺
��1��23V��Ԫ�����ڱ�λ�ڵ�______����______�壮��ҵ����V2O5ұ���������������ȼ��������û�ѧ����ʽ��ʾΪ______��
��2����Ӧ�ٵ�Ŀ����______��
��3���ù����з�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ��д���ò�������Ӧ�����ӷ���ʽ______��
��4�����ữ��H2C2O4��Һ�ζ���VO2��2SO4��Һ���Բⶨ��Ӧ�ں���Һ�к�������д����ƽ�����������ӷ���ʽ��
______VO2++______H2C2O4+______H+��______VO2++______ CO2+______��
��5���������ط�����ã�NH4VO3�ڱ��չ����У����������ļ���ֵ�������꣩���¶ȱ�
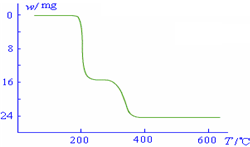 ������������ͼ��ʾ����NH4VO3�ڷֽ������______��
������������ͼ��ʾ����NH4VO3�ڷֽ������______��A���ȷֽ�ʧȥH2O���ٷֽ�ʧȥNH3
B���ȷֽ�ʧȥNH3���ٷֽ�ʧȥH2O
C��ͬʱ�ֽ�ʧȥH2O��NH3
D��ͬʱ�ֽ�ʧȥH2��N2��H2O��
�⣺��1������������Ų�ʽΪ1s22s22p63s23p63d34s2������d�����ʷ����ڵ������ڵڢ�B�壻����������������Ӧ���ɷ�������������Ӧ����ʽΪ3V2O5+10Al  6V+5Al2O3��
6V+5Al2O3��
�ʴ�Ϊ���ģ�VB��3V2O5+10Al 6V+5Al2O3��
6V+5Al2O3��
��2������������������ƣ�Ŀ��������������ԭ��Ӧ�����������ƻ�ԭV2O5����V2O5 ת��Ϊ�����Ե�VOSO4�������ᴿ��
�ʴ�Ϊ����V2O5 ת��Ϊ�����Ե�VOSO4��
��3������NH4VO3������ˮ�����ø��ֽⷴӦ����VO3-�����ӷ���ʽΪ��NH4++VO3-=NH4VO3����
�ʴ�Ϊ��NH4++VO3-=NH4VO3����
��4���ⶨ��Ӧ����Һ�з��ĺ�����������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4�����ݻ��ϼ�������д�������ӷ���Ϊ2VO2++H2C2O4+2H+=2 VO2++2 CO2��+2 H2O��
�ʴ�Ϊ��2��1��2��2��2��2H2O��
��5������NH4VO3�ڱ��ձ仯��ͼ���֪��
2NH4VO3�TV2O5+2NH3��+H2O
234g 34g 18g
����ֵ��ʼΪ0��17g�����ߵ�200��ʱ��ԼΪ16.0g�����߿�ʼƽֱ����ԼΪ300��ʱ�ֿ�ʼ���٣�H2O������������350��ʱ����24gʱ�Ͳ��ٱ仯������NH4VO3�ڱ��չ�����200��ʱ������ʧȥ������300��350����ʧȥˮ��
�ʴ�Ϊ��B��
��������1������������Ų�ʽΪ1s22s22p63s23p63d34s2���ݴ�ȷ�������ڱ���λ�ã����ȷ�Ӧʵ�����û���Ӧ������������������Ӧ���ɷ�����������
��2�������������ͼ��֪��Ӧ�ٵ�Ŀ�ģ���V2O5 ת��Ϊ�����Ե�VOSO4�����ڷ����ᴿ��
��3�����������еij���������д���ӷ���ʽ��
��4��������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4��������Ӧ�����ӷ��̸���������ԭ��Ӧ�Ļ��ϼ�����������۵ķ�Ԫ�ر���ԭΪ���ļ۵ķ�Ԫ�أ�H2C2O4������Ϊ������̼��д��������ƽ�ɵ����ӷ��̣�
��5������NH4VO3�ڱ��ձ仯��ͼ����н��
������������Ҫ������ԭ�ӷ��ŵĺ��塢���ȷ�Ӧ��Ӧ�á�������ԭ��Ӧ��ʵ�ʡ����ӷ��̵���д��ƽʱע�����֪ʶ������������������Ŀ�Ѷ��еȣ�
 6V+5Al2O3��
6V+5Al2O3���ʴ�Ϊ���ģ�VB��3V2O5+10Al
 6V+5Al2O3��
6V+5Al2O3����2������������������ƣ�Ŀ��������������ԭ��Ӧ�����������ƻ�ԭV2O5����V2O5 ת��Ϊ�����Ե�VOSO4�������ᴿ��
�ʴ�Ϊ����V2O5 ת��Ϊ�����Ե�VOSO4��
��3������NH4VO3������ˮ�����ø��ֽⷴӦ����VO3-�����ӷ���ʽΪ��NH4++VO3-=NH4VO3����
�ʴ�Ϊ��NH4++VO3-=NH4VO3����
��4���ⶨ��Ӧ����Һ�з��ĺ�����������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4�����ݻ��ϼ�������д�������ӷ���Ϊ2VO2++H2C2O4+2H+=2 VO2++2 CO2��+2 H2O��
�ʴ�Ϊ��2��1��2��2��2��2H2O��
��5������NH4VO3�ڱ��ձ仯��ͼ���֪��
2NH4VO3�TV2O5+2NH3��+H2O
234g 34g 18g
����ֵ��ʼΪ0��17g�����ߵ�200��ʱ��ԼΪ16.0g�����߿�ʼƽֱ����ԼΪ300��ʱ�ֿ�ʼ���٣�H2O������������350��ʱ����24gʱ�Ͳ��ٱ仯������NH4VO3�ڱ��չ�����200��ʱ������ʧȥ������300��350����ʧȥˮ��
�ʴ�Ϊ��B��
��������1������������Ų�ʽΪ1s22s22p63s23p63d34s2���ݴ�ȷ�������ڱ���λ�ã����ȷ�Ӧʵ�����û���Ӧ������������������Ӧ���ɷ�����������
��2�������������ͼ��֪��Ӧ�ٵ�Ŀ�ģ���V2O5 ת��Ϊ�����Ե�VOSO4�����ڷ����ᴿ��
��3�����������еij���������д���ӷ���ʽ��
��4��������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4��������Ӧ�����ӷ��̸���������ԭ��Ӧ�Ļ��ϼ�����������۵ķ�Ԫ�ر���ԭΪ���ļ۵ķ�Ԫ�أ�H2C2O4������Ϊ������̼��д��������ƽ�ɵ����ӷ��̣�
��5������NH4VO3�ڱ��ձ仯��ͼ����н��
������������Ҫ������ԭ�ӷ��ŵĺ��塢���ȷ�Ӧ��Ӧ�á�������ԭ��Ӧ��ʵ�ʡ����ӷ��̵���д��ƽʱע�����֪ʶ������������������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
�����Ŀ
���Ų��Ͽ�ѧ�ķ�չ������������Ϊ���Ͻ��ά���ء���Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ�
��֪��V���γ�VO2+��VO2+��VO3-�ȶ������ӣ����ֺ���������ˮ�е��ܽ������±���ʾ��
�ù��յ���Ҫ�������£�
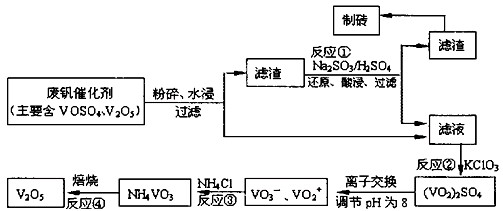
��ش���������
��1����ҵ�ϳ������ȷ�Ӧ����V2O5ұ������������д����Ӧ�Ļ�ѧ����ʽ ��
��2����Ӧ�ٵ����ӷ���ʽ�� ��
��3���ڷ�Ӧ���У�ÿ����1mol��VO2��2SO4ת�Ƶ��ӵ���ĿΪ ��
��4�������ӽ��������У��������淴ӦVO2++2OH-?VO3-+H2O��
�÷�Ӧ�Ļ�ѧƽ�ⳣ��K �ı���ʽΪ ��
��5����Ӧ�۳�ַ�Ӧ�����NH4VO3��ʵ��������Ҫ��������Ϊ ��Ϊ��֤��Ʒ���ȣ���Ӧ���еIJ��������� ��
��֪��V���γ�VO2+��VO2+��VO3-�ȶ������ӣ����ֺ���������ˮ�е��ܽ������±���ʾ��
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |

��ش���������
��1����ҵ�ϳ������ȷ�Ӧ����V2O5ұ������������д����Ӧ�Ļ�ѧ����ʽ
��2����Ӧ�ٵ����ӷ���ʽ��
��3���ڷ�Ӧ���У�ÿ����1mol��VO2��2SO4ת�Ƶ��ӵ���ĿΪ
��4�������ӽ��������У��������淴ӦVO2++2OH-?VO3-+H2O��
�÷�Ӧ�Ļ�ѧƽ�ⳣ��K �ı���ʽΪ
��5����Ӧ�۳�ַ�Ӧ�����NH4VO3��ʵ��������Ҫ��������Ϊ
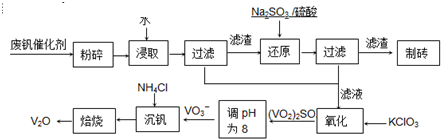
 ����a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ
����a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ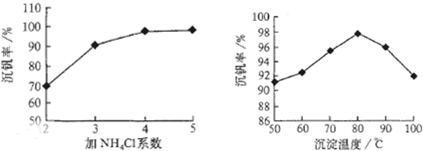
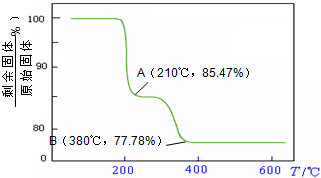 ��NH4VO3�ڷֽ������
��NH4VO3�ڷֽ������
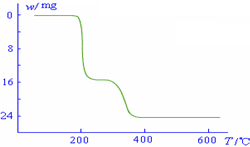 ������������ͼ��ʾ����NH4VO3�ڷֽ������
������������ͼ��ʾ����NH4VO3�ڷֽ������