��Ŀ����
17�� �����£���0.10mol•L-1����ֱ�ζ�20.00mL 0.10mol•L-1����������Һ�Ͱ�ˮ���ζ���������ҺpH������������[V��HCl��]�ı仯��ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
�����£���0.10mol•L-1����ֱ�ζ�20.00mL 0.10mol•L-1����������Һ�Ͱ�ˮ���ζ���������ҺpH������������[V��HCl��]�ı仯��ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | ���ʾ���ǵζ���ˮ�����ߣ���V��HCl��=20 mLʱ���У�c��Cl-����c��NH4+����c��H+����c��OH-�� | |
| B�� | ��pH=7ʱ���ζ���ˮ���ĵ�V��HCl��=20 mL����c��NH4+��=c��Cl-�� | |
| C�� | �ζ�����������Һʱ����V��HCl����20 mL����һ���У�c��Cl-����c��Na+����c��OH-����c��H+�� | |
| D�� | ���ζ���ˮ����V��HCl��=10 mLʱ���У�2[c��OH-��-c��H+��]=c��NH4+��-c��NH3•H2O�� |
���� A�����ݰ�ˮ��NaOH�ڵζ���ʼʱ��pH���жϣ�
B�������백ˮǡ�÷�Ӧ�����Ȼ�泥���Һ�����ԣ�
C���ζ�����������Һʱ����V��HCl����20 mL��������Ũ�ȿ��ܴ��������ӣ�
D�����ζ���ˮ����V��HCl��=10 mLʱ����Һ������Ϊ�����ʵ�����NH3•H2O��NH4Cl��
��� �⣺A��һˮ�ϰ����������Һ�в��ֵ��룬��ʼʱ��ˮ��pHС��13�����Ԣ��ʾ���ǵζ���ˮ�����ߣ���V��HCl��=20 mLʱ��ǡ�÷�Ӧ�����Ȼ�泥���Һ�����ԣ���c��Cl-����c��NH4+����c��H+����c��OH-������A��ȷ��
B���ζ���ˮ���ĵ�V��HCl��=20 mL�������백ˮǡ�÷�Ӧ�����Ȼ�泥���Һ�����ԣ���pH=7ʱ���ζ���ˮ���ĵ�V��HCl����20 mL����B����
C���ζ�����������Һʱ����V��HCl����20 mL��������Ũ�ȿ��ܴ��������ӣ�����Һ������Ũ�ȹ�ϵ����Ϊ��c��Cl-����c��H+����c��Na+����c��OH-������C����
D�����ζ���ˮ����V��HCl��=10 mLʱ����Һ������Ϊ�����ʵ�����NH3•H2O��NH4Cl������غ�Ϊc��Cl-��+c��OH-��=c��NH4+��+c��H+���������غ�Ϊ��2c��Cl-��=c��NH3•H2O��+c��NH4+������2[c��OH-��-c��H+��]=c��NH4+��-c��NH3•H2O������D��ȷ��
��ѡBC��
���� ���⿼������ϵĶ����жϺͼ��㣬��Ŀ�Ѷ��еȣ�ע�����������ʵĵ����ص��Լ�����غ㡢�����غ��Ӧ�÷���������������ѧ�������Ӧ��������

| A�� | 60��������ı���HQE�����������ȫ�������������������������������� | |
| B�� | ���ȵĴ�����Һϴ��մ�����۵�����ʱ��������Ҫ�ǻ�ѧ�仯 | |
| C�� | Ӣ�������ѧ�Ҹ�����ڡ�������ά�еĴ���Ӧ���ڹ�ѧͨ�ŷ��桱������ͻ���Գɾͣ��������2009��ŵ��������ѧ����������Ʒ�Ļ���ԭ��ΪSiO2 | |
| D�� | Һ����Һ�ȡ�Һ̬�Ȼ��ⶼ�Ƿǵ���� |
| ̼����������� | |||||
| ��� | ����ʽ | �۵� | �е� | ��ѧ���� | ��; |
| DMC |  | 4�� | 90.1�� | �dz����á�����ˮ�� ���Ժܵ� | �л��ϳ��м��� |
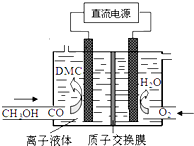 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������| A�� | ͨO2����������������ͨ������Ĥ������������������ | |
| B�� | ������Ӧ��CO-2e-+2CH3OH�T��CH3O��2CO+2H+ | |
| C�� | ����Һ�������ˮ��Һ��Ŀ���Ǵ��ݵ�� | |
| D�� | ��ϳ�DMC���ܷ�Ӧ����ʽ��CO+2CH3OH�T��CH3O��2CO+H2 |
| A�� | lmol FeI2������������Ӧʱת�Ƶĵ�����Ϊ2NA | |
| B�� | 2L 0.5 mol•L-1�������Һ�����������������ΪNA | |
| C�� | 1mol Na2O2�����к���������Ϊ3NA | |
| D�� | ��״���£�11.2L���к��з��ӵ���ĿΪ0.5NA |
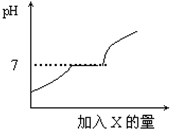 �����£���Щ����CaCl2��NaCl�����ʵ�ˮ��Һ�����ԣ���Щ����Na2CO3�����ʵ�ˮ��Һ�ʼ��ԣ���Щ����NH4Cl�����ʵ�ˮ��Һ�����ԣ�����HCl��CaCl2�Ļ����Һ����������μ������ij����X����Һ��pH�����X�����ı仯��ϵ��ͼ��ʾ����X�ǣ�������
�����£���Щ����CaCl2��NaCl�����ʵ�ˮ��Һ�����ԣ���Щ����Na2CO3�����ʵ�ˮ��Һ�ʼ��ԣ���Щ����NH4Cl�����ʵ�ˮ��Һ�����ԣ�����HCl��CaCl2�Ļ����Һ����������μ������ij����X����Һ��pH�����X�����ı仯��ϵ��ͼ��ʾ����X�ǣ�������| A�� | ˮ | B�� | ����ʯ��ˮ | C�� | ������Һ | D�� | ϡ���� |
| X | Y | |
| Z | W |
| A�� | ZW3Ϊ�Ǽ��Է��� | B�� | ԭ�Ӱ뾶��Z��X��Y | ||
| C�� | W�ĵ�������ȡ�����ԭ��֮һ | D�� | XW3��W��+1�� |
 ��O
��O ��Ca
��Ca ��
��
 ���ṹ
���ṹ 

 ��
��