��Ŀ����
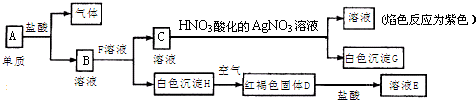
��������A��F��������Ϥ�ĵ��ʻ������A��B�dz����Ľ�����E�ڳ������ǻ���ɫ���壻���ʵ��������£�����֮����Է�����ͼ��ʾ��ת����

�Իش��������⣺
��1��A�Ļ�ѧʽ��______��
��2���ڢ�-�߷�Ӧ�У�����������ԭ��Ӧ����______�����������û���Ӧ����______��
��3��д����ͼ�а�ɫ������NaOH��Һ��Ӧ�����ӷ���ʽ��______��
��4����д����D����Һ�еμ�NaOH��Һ�Ĺ����з�����Ӧ�Ļ�ѧ����ʽ______��______��
��5��������ͼ��ɫ��Һ�н��������ӵķ����ǣ���дʵ�����ƣ���______��������______��

�Իش��������⣺
��1��A�Ļ�ѧʽ��______��
��2���ڢ�-�߷�Ӧ�У�����������ԭ��Ӧ����______�����������û���Ӧ����______��
��3��д����ͼ�а�ɫ������NaOH��Һ��Ӧ�����ӷ���ʽ��______��
��4����д����D����Һ�еμ�NaOH��Һ�Ĺ����з�����Ӧ�Ļ�ѧ����ʽ______��______��
��5��������ͼ��ɫ��Һ�н��������ӵķ����ǣ���дʵ�����ƣ���______��������______��
A�dz����Ľ�������A+�����C+H2��CΪ�����Ȼ��C��KOH��Ӧ���ɰ�ɫ��������������KOH�����ᣬ�ʸð�ɫ����ΪAl��OH��3����CΪAlCl3��AΪAl��E�ڳ������ǻ���ɫ���壬EΪCl2����B+�����D+H2��֪��DΪ�����Ȼ��D����������Ӧ����F��F�������B��Ӧ�õ�D����BΪ��۽���Fe����DΪFeCl2��FΪFeCl3��FeCl3��Һ������Fe��Ӧ����FeCl2��
��1��������������֪��A�Ļ�ѧʽ��Al���ʴ�Ϊ��Al��
��2���ڢ�-�߷�Ӧ�У���Ӧ�٢ڢݢ�����������ԭ��Ӧ�����Т٢������û���Ӧ��
�ʴ�Ϊ���٢ڢݢޣ��٢ڣ�
��3��ͼ�а�ɫ����Ϊ������������NaOH��Һ��Ӧ����ƫ��������ˮ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��4��FeCl2��Һ�еμ�NaOH��Һ�Ĺ����з�����Ӧ�Ļ�ѧ����ʽ��FeCl2+2NaOH=Fe��OH��2��+2NaCl��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ��FeCl2+2NaOH=Fe��OH��2��+2NaCl��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��5����ͼ��ɫ��Һ�н���������ΪK+��������ɫ��Ӧ���м��飬����ɫ�ܲ����۲������ɫΪ��ɫ˵������K+��
�ʴ�Ϊ����ɫ��Ӧ������ɫ�ܲ����۲������ɫΪ��ɫ��
��1��������������֪��A�Ļ�ѧʽ��Al���ʴ�Ϊ��Al��
��2���ڢ�-�߷�Ӧ�У���Ӧ�٢ڢݢ�����������ԭ��Ӧ�����Т٢������û���Ӧ��
�ʴ�Ϊ���٢ڢݢޣ��٢ڣ�
��3��ͼ�а�ɫ����Ϊ������������NaOH��Һ��Ӧ����ƫ��������ˮ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��4��FeCl2��Һ�еμ�NaOH��Һ�Ĺ����з�����Ӧ�Ļ�ѧ����ʽ��FeCl2+2NaOH=Fe��OH��2��+2NaCl��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ��FeCl2+2NaOH=Fe��OH��2��+2NaCl��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��5����ͼ��ɫ��Һ�н���������ΪK+��������ɫ��Ӧ���м��飬����ɫ�ܲ����۲������ɫΪ��ɫ˵������K+��
�ʴ�Ϊ����ɫ��Ӧ������ɫ�ܲ����۲������ɫΪ��ɫ��

��ϰ��ϵ�д�
�����Ŀ