题目内容
新制氯水中含有多种粒子,某校化学研究性学习小组的同学为探究其性质,做了如下实验,请你帮助完成:
(1)氯气能使湿润的红色布条褪色。使其褪色的微粒的化学式是______
(2)将氯水在光照上一段时间,溶液颜色逐渐变浅,其有关反应的化学方程式为:
、
(3)平衡常数表明了封闭体系的可逆反应在给定的温度下进行的程度,对于同一个类型的反应,平衡常数越大,表明反应进行的程度越大。
H2CO3 ![]()
![]() + H+ Ka1(H2CO3)=4.45×10—7
+ H+ Ka1(H2CO3)=4.45×10—7
![]()
![]()
![]() +H+ Ka2(HCO3-)=5.61×10-11
+H+ Ka2(HCO3-)=5.61×10-11
HclO ![]() H++
H++![]() Ka(HClO)=2.95×10-8
Ka(HClO)=2.95×10-8
请依据以上电离平衡常数,请写出将少量的氯气通入到过量的碳酸钠溶液中所发生反应的离子方程式:
(4)饱和氯水与石灰石的反应是制取较浓HClO溶液的方法之一。

实验一、定性研究:
在试管中加入过量的块状碳酸钙,再加入约20mL饱和氯水,充分反应,
有少量气泡产生,溶液浅黄绿色褪去;
② 过滤,将滤液滴在有色布条上,发现其比氯水的漂白性更强;
③ 为了确定反应产物,将滤液分为三份,分别进行以下实验:
第一份与石灰水混合,立即产生大量白色沉淀;
第二份与稀盐酸混合,立即产生大量无色气体;
将第三份加热,看到溶液变浑浊且有大量无色气体产生。
经检测,上述实验中产生的无色气体均为CO2。
请回答:
① 反应后所得的溶液漂白性增强的原因是______ ___________ ____
②依据上述实验可推知:②的滤液中的溶质除CaCl2、HClO外,还含有______ _ 。
实验二、定量研究:
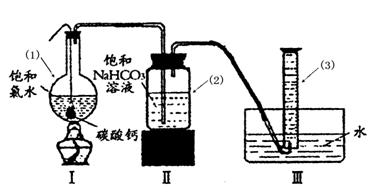
在圆底烧瓶底部,有一用塑料网包住的过量块状 碳酸钙和150mL饱和氯水,按如图所示装置实验,待不再产生气泡后,将塑料网中剩余的石灰石提出液面,密封后再加热、煮沸烧瓶中的液体,直到量筒中的气体不再增加(不考虑HClO的分解)。
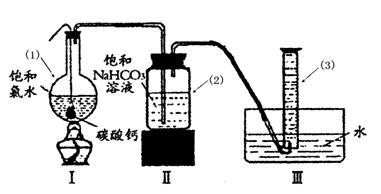
请回答:
③ 写出仪器(1)与仪器(3)的名称
(1) (3)
④ 为减少用装置Ⅲ收集气体过程中CO2因溶解而造成的损失,且水槽中仍然为水,请你对装置Ⅲ进行改进,最简单的方法是 。
⑤ 如何准确读出量筒中气体的体积
a________ _
b 上下移动量筒至量筒内液面与水槽液面相平
c
(1)![]() (1分)
(1分)
(2)![]() +
+![]()
![]()
![]() +
+![]() (1分)
(1分)
2![]()
![]() 2
2![]() +
+![]() (1分)
(1分)
(3)![]() +2
+2![]() +
+![]()
![]() 2
2![]() +
+![]() +
+![]() (2分)
(2分)
(4)①![]() 消耗了氯水中的
消耗了氯水中的![]() ,使
,使![]() +
+![]()
![]()
![]() +
+![]() 平衡向右移动
平衡向右移动 ![]() 浓度增大(2分)
浓度增大(2分)
②![]() (写名称也对)2分
(写名称也对)2分
③(1)圆底烧瓶 (1分) (3)量筒(1分)
④在导管末端再连接长导管,使导管的出口接近量筒底部(2分)
⑤a烧瓶冷却至室温 (1分) c平视刻度线读数(1分)
解析:
略

 ②过滤,将滤液滴在有色布条上,发现其比氯水的漂白性更强;
②过滤,将滤液滴在有色布条上,发现其比氯水的漂白性更强;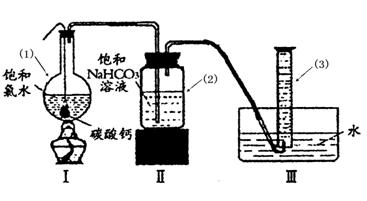
 越大。
越大。
 + H+ Ka1(H2CO3)=4.45×10—7
+ H+ Ka1(H2CO3)=4.45×10—7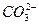 +H+ Ka2(HCO3-)=5.61×10-11
+H+ Ka2(HCO3-)=5.61×10-11 Ka(HClO)=2.95×10-8
Ka(HClO)=2.95×10-8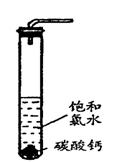
 面,密封后再加热、煮沸烧瓶中的液体,直到量筒中的气体不再增加(不考虑HClO的分解)。
面,密封后再加热、煮沸烧瓶中的液体,直到量筒中的气体不再增加(不考虑HClO的分解)。