��Ŀ����
������ˮ�к��ж������ӣ�ijУ��ѧ�о���ѧϰС���ͬѧΪ̽�������ʣ���������ʵ�飬���������ɣ�
��1��HClO���ȶ��������ֽ�����HCl��O2�������ʵ��֤����O2���ɣ�
��2��������ʹʪ��ĺ�ɫ������ɫ������ʹ��ɫ������ɫ�����ʣ�ͬѧ�ǵĿ�����һ�£���Ϊ��ˮ�д��ڵļ������Ӷ��п��ܣ��������ʵ�飬�ó���ȷ���ۣ�
������⣺��ˮ�к���������ʹʪ��ĺ�ɫ������ɫ
�ռ����ϣ���������ǿ�����ԣ�����������ˮ��Ӧ��������ʹ�����۴�������ǿ������
������裺
��
��
��
��H2Oʹ������ɫ
��3����֤���裺ʵ��٣��Ѻ�ɫ�ɲ���������������ļ���ƿ�У���������ɫ
ʵ��ڣ�
ʵ��ۣ�
ʵ��ܣ��Ѻ�ɫ��������ˮ�ﲼ������ɫ
�ó����ۣ�
��1��HClO���ȶ��������ֽ�����HCl��O2�������ʵ��֤����O2���ɣ�
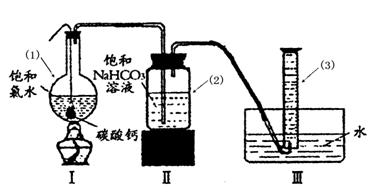
��ʢ����ˮ����ɫ����ƿ������ˮ���У�����һ��ʱ�������ɫ�������ɣ���ƿ�ӵ�ת�������ô����ǵ�ľ������ƿ�ڼ������壬��ľ����ȼ��֤��HClO�ֽ����ɵ�����Ϊ����
��ʢ����ˮ����ɫ����ƿ������ˮ���У�����һ��ʱ�������ɫ�������ɣ���ƿ�ӵ�ת�������ô����ǵ�ľ������ƿ�ڼ������壬��ľ����ȼ��֤��HClO�ֽ����ɵ�����Ϊ����
����2��������ʹʪ��ĺ�ɫ������ɫ������ʹ��ɫ������ɫ�����ʣ�ͬѧ�ǵĿ�����һ�£���Ϊ��ˮ�д��ڵļ������Ӷ��п��ܣ��������ʵ�飬�ó���ȷ���ۣ�
������⣺��ˮ�к���������ʹʪ��ĺ�ɫ������ɫ
�ռ����ϣ���������ǿ�����ԣ�����������ˮ��Ӧ��������ʹ�����۴�������ǿ������
������裺
��
����ʹ������ɫ
����ʹ������ɫ
����
������ʹ������ɫ
������ʹ������ɫ
����
����ʹ������ɫ
����ʹ������ɫ
����H2Oʹ������ɫ
��3����֤���裺ʵ��٣��Ѻ�ɫ�ɲ���������������ļ���ƿ�У���������ɫ
ʵ��ڣ�
�Ѻ�ɫ����������ˮ�У���ɫ������ɫ
�Ѻ�ɫ����������ˮ�У���ɫ������ɫ
ʵ��ۣ�
�Ѻ�ɫ��������ϡ�����У���ɫ��������ɫ
�Ѻ�ɫ��������ϡ�����У���ɫ��������ɫ
ʵ��ܣ��Ѻ�ɫ��������ˮ�ﲼ������ɫ
�ó����ۣ�
������ʹ��ɫ������ɫ
������ʹ��ɫ������ɫ
����������1��������ʹ�����ǵ�ľ����ȼ��
��2��������ˮ�ijɷֽ��м��裬��ˮ�к��������������ᡢ�����ˮ��Ȼ�����ν��м��裻
��3�����öԱ�ʵ����֤���裬����ʵ�����������жϣ��Ӷ��ó����ۣ�
��2��������ˮ�ijɷֽ��м��裬��ˮ�к��������������ᡢ�����ˮ��Ȼ�����ν��м��裻
��3�����öԱ�ʵ����֤���裬����ʵ�����������жϣ��Ӷ��ó����ۣ�
����⣺��1��������ȶ��������ֽ�������������ʢ����ˮ����ɫ����ƿ������ˮ���У�����һ��ʱ��ῴ�������ݲ�������ƿ�ӵ�ת�������ô����ǵ�ľ������ƿ�ڼ������壬���ľ����ȼ��˵�����ȷֽ������������������
�ʴ�Ϊ����ʢ����ˮ����ɫ����ƿ������ˮ���У�����һ��ʱ�������ɫ�������ɣ���ƿ�ӵ�ת�������ô����ǵ�ľ������ƿ�ڼ������壬��ľ����ȼ��֤��HClO�ֽ����ɵ�����Ϊ������
��2����������ˮ������ӦCl2+H2O?HCl+HClO�����ݷ���ʽ֪����Һ�еijɷ���������ˮ������ʹ����ᣬ������ˮ�еijɷֽ��м��裺
������ʹ������ɫ��
��HClOʹ������ɫ��
������ʹ������ɫ��
�ʴ�Ϊ������ʹ������ɫ��������ʹ������ɫ������ʹ������ɫ��
��3��[��֤����]
�ڰѺ�ɫ����������ˮ�У���ɫ������ɫ��
�۰Ѻ�ɫ��������ϡ�����У���ɫ��������ɫ��
���öԱ�ʵ��֪��ֻ�м�����ˮ�е���ɫ������ɫ���Ӷ��ó�����Ϊ��������ʹ��ɫ������ɫ��
�ʴ�Ϊ���Ѻ�ɫ����������ˮ�У���ɫ������ɫ���Ѻ�ɫ��������ϡ�����У���ɫ��������ɫ��������ʹ������ɫ��
�ʴ�Ϊ����ʢ����ˮ����ɫ����ƿ������ˮ���У�����һ��ʱ�������ɫ�������ɣ���ƿ�ӵ�ת�������ô����ǵ�ľ������ƿ�ڼ������壬��ľ����ȼ��֤��HClO�ֽ����ɵ�����Ϊ������
��2����������ˮ������ӦCl2+H2O?HCl+HClO�����ݷ���ʽ֪����Һ�еijɷ���������ˮ������ʹ����ᣬ������ˮ�еijɷֽ��м��裺
������ʹ������ɫ��
��HClOʹ������ɫ��
������ʹ������ɫ��
�ʴ�Ϊ������ʹ������ɫ��������ʹ������ɫ������ʹ������ɫ��
��3��[��֤����]
�ڰѺ�ɫ����������ˮ�У���ɫ������ɫ��
�۰Ѻ�ɫ��������ϡ�����У���ɫ��������ɫ��
���öԱ�ʵ��֪��ֻ�м�����ˮ�е���ɫ������ɫ���Ӷ��ó�����Ϊ��������ʹ��ɫ������ɫ��
�ʴ�Ϊ���Ѻ�ɫ����������ˮ�У���ɫ������ɫ���Ѻ�ɫ��������ϡ�����У���ɫ��������ɫ��������ʹ������ɫ��
���������⿼����ʵ��̽�������ݻ�����еijɷֽ��м��裬���ʵ����֤���裬���ݶԱ�ʵ���в�ͬ����ó����ۣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ
 �ڹ��ˣ�����Һ������ɫ�����ϣ����������ˮ��Ư���Ը�ǿ��
�ڹ��ˣ�����Һ������ɫ�����ϣ����������ˮ��Ư���Ը�ǿ��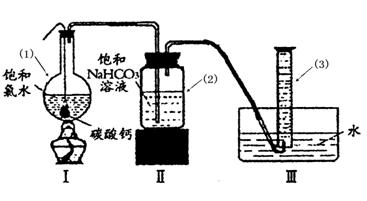
 Խ��
Խ��
 �� H�� Ka1��H2CO3��=4.45��10��7
�� H�� Ka1��H2CO3��=4.45��10��7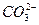 ��H�� Ka2(HCO3��)=5.61��10��11
��H�� Ka2(HCO3��)=5.61��10��11 Ka(HClO)=2.95��10��8
Ka(HClO)=2.95��10��8
 �棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��
�棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��