��Ŀ����
����Ŀ�����ú�����Դ���Ի�úܶ����ʡ���Ӻ�ˮ�п��Եõ�ʳ�ε������ԭ�ϣ�����ͨ�������õ�ˮ���Ӻ���ֲ������ȡ��ȡ�
(һ)�Ӻ�ˮ�õ��Ĵ����г�����������Ҫ�����ᴿ���ڳ�ȥ���������ɳ֮��Ҫ�������Լ������ᡢ��Na2CO3����NaOH����BaCl2����ȥʳ��ˮ�е�Ca2+�� Mg2+��SO42-��
(1)Ϊ��Ч��ȥCa2+�� Mg2+��SO42-�������Լ��ĺ���˳��Ϊ___________��
a.�ȼ� NaOH�����Na2CO3���ټ� BaCl2
b.�ȼ�NaOH�����BaCl2���ټ�Na2CO3
C.�ȼ�BaCl2�����NaOH���ټ�Na2CO3
(2)����Na2CO3�����з�����Ӧ�����ӷ���ʽΪ_______________________��
(3)�ж��Լ�BaCl2���������ķ�����_______________________��
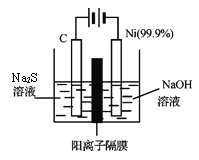
(��������ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ����������£�
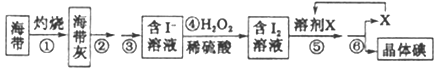
(1)ʵ��ʱ���պ���Ӧ��___________(����������)�ڽ��С�
(2)������з�Ӧ�����ӷ���ʽ��_______________��
(3)����ݵ�ʵ�����Ϊ____________��Ӧѡ�õ�һ���ܼ�X������____________��
a.�����ƾ� b.���Ȼ�̼���� c.���͡��ƾ�
(4)�����ʵ�����Ӧѡ����ͼ�е�_____________��
���𰸡� bc Ba2++ CO32-=BaCO3�� ��Ca2- +CO32-=CaCO3�� ���ã����ϲ�[Һ�еμ�BaCl2��Һ�������ٲ�����ɫ��������BaCl2������ ���� 2I++H2O2+2H+=I2+2H2O ��ȡ��Һ b C
��������(һ)(1)Ca2����̼���Ƴ�ȥ��Mg2�����������Ƴ�ȥ��SO42�����Ȼ�����ȥ�������������ữ�������ڹ������Ȼ���Ҫ��̼��������������̼���Ʊ�������Ȼ����ĺ��棬���������ƿ������������ѡ��bc��ȷ��(2)�������Ϸ�����֪����Na2CO3�����з�����Ӧ�����ӷ���ʽΪ Ba2++ CO32-=BaCO3����Ca2- +CO32-=CaCO3����(3)�ж�BaCl2�ѹ����ķ����Ǽ��������Ȼ�����Һ����ȡ����������ϲ���Һ1��2���ڵ�ΰ��ϣ���ȡ�����ϲ���Һ���Թ��У����ٵ���1��2��BaCl2��Һ������Һδ����ǣ������BaCl2�ѹ�����
(����(1)ʵ��ʱ���պ���Ӧ�������ڽ��С�(2)�������˫��ˮ���������ӣ���Ӧ�����ӷ���ʽ��2I++H2O2+2H+=I2+2H2O��(3)���������л��ܼ��У���˲���ݵ�ʵ�����Ϊ��ȡ��Һ���ƾ���ˮ���ܣ������п�����ⷴӦ����˿���ѡ�����Ȼ�̼������ȡ������ѡb��(4)�������������װ��ͼ���жϷֱ��ǹ��ˡ�����������ͷ�Һ����ѡC��

����Ŀ���״����Ҵ��������г�������;�㷺�����ʣ���ϳɷ��������ʾ������о���ֵ��
(1)��֪�±��м������ݣ�����̬�Ҵ���ȫȼ������CO2��ˮ�������Ȼ�ѧ����ʽΪ__________��
��ѧ�� | C-C | C-H | O-O | H-O | C-O | C-O |
����/(kJ��mol-1) | 348 | 413 | 498 | 463 | 351 | 799 |
(2)��һ�ݻ��ɱ���ܱ������г���1mol CO��2 molH2��������Ӧ��CO(g)+2H2(g)![]() CH3OH(g) ��H1<0��CO�ڲ�ͬ�¶��µ�ƽ��ת����(a)��ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g) ��H1<0��CO�ڲ�ͬ�¶��µ�ƽ��ת����(a)��ѹǿ�Ĺ�ϵ��ͼ��ʾ��
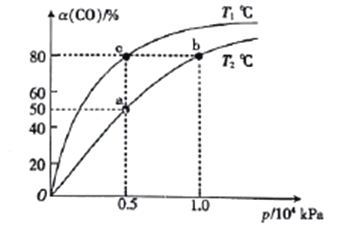
��a��b����ķ�λ���ʣ�v(b)_____v(a)(�>����<����=������ͬ)��
��T1____T2��
�۸úϳɷ�Ӧ���¶�һ�������240��270�棬ѡ��˷�Χ��ԭ���¶ȷ�Χ�µĴ������Ըߣ�__________________________��
��ͼ��a��b��c�����Ӧ�Ļ�ѧƽ�ⳣ��K(a)��K(b)��K(c)�Ĵ�С��ϵΪ_________��
(3)���úϳ���(��Ҫ�ɷ�ΪCO��H2)�ϳɼ״�����Ҫ�������·�Ӧ��
CO(g)+2H2(g) ![]() CH3OH(g) ��H1��
CH3OH(g) ��H1��
CO2(g)+H2(g) ![]() CO(g)+H2O(g) ��H2��
CO(g)+H2O(g) ��H2��
CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H3��
CH3OH(g)+H2O(g) ��H3��
������Ӧ��Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2��K3������K1��K2���¶ȵı仯��ͼ��ʾ��

���H1_____((�>����<����=��) ��H3��������__________________��