��Ŀ����
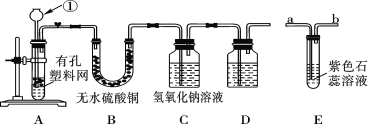
����Ŀ����ͼ�����ڼ��л���������Ʊ������롢�ᴿ�����ļ���װ�á�����ݸ�װ�ûش��������⣺

(1)����C3H8O�����������ᷴӦ����������������ƿA�м���C3H8O�������������⣬��Ӧ������Լ��� ___________���Թ�B��Ӧ���� ____________������̼������Һ���߿��еĵ��������� ______________.
(2)����ʽΪC3H8O���л�����һ����������ˮ���ɵ��л�������� ________�֣�
(3)���ø�װ�÷��������1-������������ƿA�м���1-�����������⣬��Ӧ�ȼ����������Լ� ____���ѧʽ�������ȵ�һ���¶ȣ��Թ�B���ռ������ǣ���д�ṹ��ʽ�� _____����ȴ��������ƿ�м����Լ� ______ �������ƣ����ټ��ȵ�һ���¶ȣ��Թ�B���ռ������� _____����д����ʽ����
���𰸡�Ũ���� ����̼������Һ ���������� 4 CaO CH3CH2CH2OH Ũ���� C2H4O2
��������
(1)������ӦҪ��Ũ�������������ñ���̼������Һ���������������������������������������ã�
(2)���ݷ���ʽȷ���������࣬�����ܷ�����������ˮ�ͷ��Ӽ���ˮ��
(3)�Ȱ���ת��Ϊ����Һ�����������Ϊ������ʣ���ˮ��Һ�м�����õ����ᣬ��ͨ���������.
(1)C3H8O(��)�����ᷢ��������ӦҪ��Ũ���������������ɵ�������������к��лӷ��ı��������ᣬҪ�ñ���̼������Һ���������������������������������������ã�
(2)C3H8O��Ӧ�Ĵ������֣�CH3CH2CH2OH��CH3CHOHCH3�����ִ��ɷ�����ȥ��Ӧ����ͬһ�����ʱ�ϩ�����������ӵĴ��ɷ������Ӽ���ˮ��Ӧ�����ѣ���������ͬ�����ӣ�Ҳ�����Dz�ͬ�����ӣ������ִ�������ϣ�������3���ѣ��������ɵ��л��������4�֣�
(3)�������1-�����Ļ��Һ�м���CaO��ʹ������CaO��Ӧ��������ƣ�����ת��Ϊ����Һ�����������ӻ�����е�ߣ������������������Թ�B���ռ��������Ϊ��������ƿ��ʣ���Ϊ����Ƶ�ˮ��Һ��Ȼ�����������Һ�м�Ũ���ᣬ�������ֽⷴӦ�������������ƣ�Ȼ��ͨ�������ɷ���õ����ᡣ

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д�����Ŀ��ij�ϳ�������Ҫ�ɷ���һ����̼�������������ںϳɼ���(CH3OCH3)�����ȼ�ϡ�����Ȼ����øúϳ��������п��ܷ����ķ�Ӧ�У�
��CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H1��+206.1 kJ��mol-1
CO(g)+3H2(g) ��H1��+206.1 kJ��mol-1
��CH4(g)+CO2(g)![]() 2CO(g)+2H2(g) ��H2��+247.3 kJ��mol-1
2CO(g)+2H2(g) ��H2��+247.3 kJ��mol-1
��CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H3
CO2(g)+H2(g) ��H3
��ش��������⣺
��1����һ�ܱ������н��з�Ӧ�������CH4�����ʵ���Ũ���淴Ӧʱ��ı仯��ͼ��ʾ������10 minʱ���ı���������������__________________________��

��2����Ӧ������H3��_____________��800 ��ʱ����Ӧ����ƽ�ⳣ��K��1����ø��¶����ܱ�������ijʱ�̸����ʵ����ʵ������±���
CO | H2O | CO2 | H2 |
0.5 mol | 8.5 mol | 2.0 mol | 2.0 mol |
��ʱ��Ӧ���������淴Ӧ���ʵĹ�ϵʽ��_________(�����)��
a��v��>v�� b��v��<�� c��v����v�� d�����ж�
��3����ͼ2��ʾ���ڼס����������зֱ��������ʵ�����CH4��CO2��ʹ�ס�����������ʼ�ݻ���ȡ�����ͬ�¶��·�����Ӧ������ά�ַ�Ӧ�������¶Ȳ��䡣��֪��������CH4��ת������ʱ��ı仯��ͼ3��ʾ������ͼ3�л�����������CH4��ת������ʱ��仯��ͼ��___________

��4��ij�ϳ�������Ҫ�ɷ��е�һ����̼����һ��������Ҳ����NaOH��Һ��CO��Ӧ���ɼ�����(HCOONa)����һ����Ӧ���ɼ���������CO��Ⱦ�������½�a mol��COͨ��2 L b mol��L-1NaOH��Һ�У�ǡ����ȫ��Ӧ���ɼ����ƺͺ���������Ļ����Һ(������Һ�������)�������Һ��c(Na+)=c(HCOO-)����û����Һ�м���ĵ���ƽ�ⳣ��Ka=_________(�ú�a��b�Ĵ���ʽ��ʾ)��