��Ŀ����
����Ŀ��Cu2+����NH3��H2O��OH-��Cl-���γ���λ��Ϊ4������
��1����CuSO4��Һ�м������NaOH��Һ������Na2[Cu(OH)4]��
�ٻ���������[Cu(OH)4]2-�е���λ��__________��
��Na2[Cu(OH)4]�г�����λ���⣬�����ڵĻ�ѧ��������______(�����)��
A�����Ӽ� B��������
C�����Թ��ۼ� D���Ǽ��Թ��ۼ�
��2������ͭ�백ˮ��������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ������Ӧ:Cu+H2O2+4NH3![]() [Cu(NH3)4]2++2OH-����ԭ����_________________��
[Cu(NH3)4]2++2OH-����ԭ����_________________��
��3��Cu2+�������Ҷ���(H2N��CH2CH2��NH2)�γ�������:
 ��
��
��H��O��N����Ԫ�صĵ縺�ԴӴ�С��˳��Ϊ___________��
���Ҷ���������Nԭ�ӳɼ�ʱ��ȡ���ӻ�������__________��
���𰸡�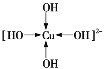 AC ��������Ϊ����������Cu����ΪCu2+����������Cu2+�γ���λ�� O>N>H sp3
AC ��������Ϊ����������Cu����ΪCu2+����������Cu2+�γ���λ�� O>N>H sp3
��������
��1����Cu2+���пչ����OH-���й¶Ե��ӣ����γ���λ����
��Na2[Cu(OH)4]�г�����λ���⣬���������Ӽ��ͼ��Թ��ۼ���
��2���������������Cu����Cu2+�������백�����γ���λ����
��3�������ݵ縺�������ڱ��еݱ���ɷ�����
���Ҷ���������Nԭ���γ�4��������
��1����Cu2+���пչ����OH-���й¶Ե��ӣ����γ���λ����������[C u(OH)4]2+��1��Cu2+��4��OH-�γ���λ�����ɱ�ʾΪ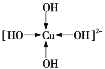 ��
��
��Na2[Cu(OH)4]Ϊ���ӻ�����������Ӽ�������O-HΪ���Թ��ۼ����ʴ�ΪAC��
��2���������������Cu����Cu2+�������백�����γ���λ����������ٽ�ʹ��Ӧ�������ʴ�Ϊ��������Ϊ����������Cu����ΪCu2+����������Cu2+�γ���λ����
��3����ͬ����Ԫ�ش�����Ԫ�صĵ縺������ǿ����O��N��H�ĵ縺������������O��N��H��
���Ҷ���������Nԭ���γ�4��������Ϊsp3�ӻ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�