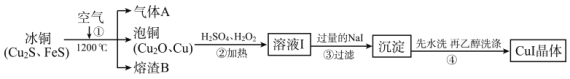
��Ŀ����
����Ŀ���л���A��̼���⡢������Ԫ����ɣ����������Ƿ��͵õ���Ҳ�ɴ���ţ������ȡ��������AΪ��ɫճ��Һ�壬������ˮ��Ϊ�о�A�������ṹ������������ʵ�飺
ʵ�鲽�� | ʵ����� |
��1����ȡ�л���A 9.0g������ʹ�������������ܶ�����ͬ������H2��45����A���ӵ�����ͼ����ͼ��ʾ��
| ��1���л���A��Ħ������Ϊ______�� |
��2������9.0g A��������O2���ȼ�գ���ʹ ���������ͨ����ˮ�Ȼ��ơ���ˮ����ͭ����ʯ�ҡ�ʵ�������ͭ��ĩû�б�����������ˮ�Ȼ�������5.4g����ʯ������13.2g�� | ��2�������㣬�л���A�ķ���ʽΪ_______�� |
��3����������ײⶨ��֤ʵ���к���-OH�ͣ�COOH���ֹ����ţ����˴Ź������IJⶨ����˴Ź�������ͼ����ͼ��
| ��3��A�Ľṹ��ʽ____________________�� |
��4����������ײⶨ��A��һ��ͬ���칹��B�У�������A��ͬ�Ĺ����š� | ��4��A��ͬ���칹��B�Ľṹ��ʽΪ��____�� |
���𰸡�90 g/molC3H6O3![]() CH2(OH)-CH2-COOH
CH2(OH)-CH2-COOH
��������
��1���л����ʵ��ܶ�����ͬ������H2��45���������л����ʵ�Ħ������=45g/mol��2=90g/mol����ˣ�������ȷ���ǣ�90g/mol��
��2��9.0gA�����ʵ���=9/90=0.1mol��Ũ��������5.4g����n��H2O��=5.4/18=0.3mol��������n��H��=0.6mol����ʯ������13.2g����n��CO2��=13.2/44=0.3mol�����Է�����N��C��=0.3/0.1=3��N��H��=��0.3��2��/0.1=6���ʷ�����N��O��=��90-12��3-6��/16=3����A�ķ���ʽΪ��C3H6O3����ˣ�������ȷ���ǣ�C3H6O3��
��3�����ݺ˴Ź�������ͼ�����л�������4����ֵ����4�����͵ĵ�Ч��ԭ�ӣ���ԭ�ӵĸ�������3��1��1��1����A����һ���Ȼ���һ���ǻ������Խṹ��ʽΪ��![]() ���������������������
���������������������![]() ��
��
��4��A�Ľṹ��ʽΪ![]() ����������ײⶨ��A��һ��ͬ���칹��B�У�������A��ͬ�Ĺ������������л���B�Ľṹ��ʽΪCH2(OH)-CH2-COOH ���������������������CH2(OH)-CH2-COOH��
����������ײⶨ��A��һ��ͬ���칹��B�У�������A��ͬ�Ĺ������������л���B�Ľṹ��ʽΪCH2(OH)-CH2-COOH ���������������������CH2(OH)-CH2-COOH��

 ��У����ϵ�д�
��У����ϵ�д�