ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩν―±Μ≥ΤΈΣΦΧΧζΓΔ¬Ν÷°ΚσΒΡΒΎ»ΐΫπ τΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)ΫπΚλ ·(TiO2) «ν―ΒΡ÷ς“ΣΩσΈο÷°“ΜΘ§ΜυΧ§Ti‘≠Ή”Φέ≤ψΒγΉ”ΒΡ≈≈≤ΦΆΦΈΣ_________Θ§ΜυΧ§O‘≠Ή”ΒγΉ”’ΦΨίΉνΗΏΡήΦΕΒΡΒγΉ”‘Τ¬÷άΣΆΦΈΣ __________–ΈΓΘ
(2)“‘TiO2ΈΣ‘≠ΝœΩ…÷ΤΒΟTiCl4Θ§TiCl4ΒΡ»έΓΔΖ–ΒψΖ÷±πΈΣ205KΓΔ409KΘ§ΨυΗΏ”ΎΫαΙΙ”κΤδœύΥΤΒΡCCl4Θ§÷ς“Σ‘≠“ρ « __________________ΓΘ
(3)TiCl4Ω…»ή”Ύ≈®―ΈΥαΒΟH2[TiCl6]Θ§œρ»ή“Κ÷–Φ”»κNH4Cl≈®»ή“ΚΩ…Έω≥ωΜΤ…ΪΒΡ(NH4)2[TiCl6]ΨßΧεΓΘΗΟΨßΧε÷–ΈΔΙέΝΘΉ”÷°ΦδΒΡΉς”ΟΝΠ”– ________ΓΘ
AΘ°άκΉ”Φϋ BΘ°Ι≤ΦέΦϋ CΘ°Ζ÷Ή”ΦδΉς”ΟΝΠ DΘ°«βΦϋ EΘ°ΖΕΒ¬ΜΣΝΠ
(4)TiCl4Ω…”κCH3CH2OHΓΔHCHOΓΔCH3OCH3Β»”–Μζ–ΓΖ÷Ή”–Έ≥…Φ”ΚœΈοΓΘ…œ ω»ΐ÷÷–ΓΖ÷Ή”÷–C‘≠Ή”ΒΡVSEPRΡΘ–Ά≤ΜΆ§”ΎΤδΥϊΖ÷Ή”ΒΡ « _____Θ§ΗΟΖ÷Ή”÷–CΒΡΙλΒά‘”Μ·άύ–ΆΈΣ________ ΓΘ
(5)TiO2”κBaCO3“ΜΤπ»έ»ΎΩ…÷ΤΒΟν―Υα±ΒΓΘ
ΔΌBaCO3÷–“θάκΉ”ΒΡΝΔΧεΙΙ–ΆΈΣ ________ΓΘ
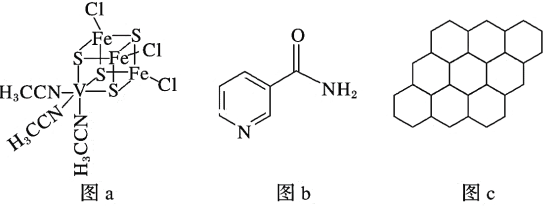
ΔΎΨ≠X…δœΏΖ÷ΈωΦχΕ®Θ§ν―Υα±ΒΒΡΨßΑϊΫαΙΙ»γœ¬ΆΦΥυ ΨΘ®Ti4+ΓΔBa2+Ψυ”κO2Θ≠œύΫ”¥ΞΘ©Θ§‘ρν―Υα±ΒΒΡΜ·―ß ΫΈΣ _________ΓΘ“―÷ΣΨßΑϊ±Ώ≥ΛΈΣa pmΘ§O2Θ≠ΒΡΑκΨΕΈΣb pmΘ§‘ρTi4+ΓΔBa2+ΒΡΑκΨΕΖ÷±πΈΣ____________pmΓΔ___________pmΓΘ

ΓΨ¥πΑΗΓΩ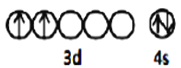 ―ΤΝε–Ά TiCl4ΒΡœύΕ‘Ζ÷Ή”÷ ΝΩ¥σ”ΎCCl4Θ§Ζ÷Ή”ΦδΉς”ΟΝΠΗϋ¥σ AB HCHO sp2 ΤΫΟφ»ΐΫ«–Έ BaTiO3
―ΤΝε–Ά TiCl4ΒΡœύΕ‘Ζ÷Ή”÷ ΝΩ¥σ”ΎCCl4Θ§Ζ÷Ή”ΦδΉς”ΟΝΠΗϋ¥σ AB HCHO sp2 ΤΫΟφ»ΐΫ«–Έ BaTiO3 ![]()
![]()
ΓΨΫβΈωΓΩ
(1)ΗυΨί‘ΣΥΊΚΥΆβΒγΉ”≈≈≤ΦΙφ¬… ι–¥ΒγΉ”≈≈≤Φ ΫΘ§ΗυΨίΒγΉ”≈≈≤Φ Ϋ≈–ΕœΉνΗΏΡήΦΕΦΑΒγΉ”‘Τ¬÷άΣΘΜ
(2)Ζ÷Ή”ΨßΧεΒΡ»έΖ–Βψ”κΖ÷Ή”ΦδΉς”ΟΝΠ”–ΙΊΘ§ΗυΨίœύΕ‘Ζ÷Ή”÷ ΝΩΖ÷Έω≈–ΕœΘΜ
(3)ΫαΚœΨßΧεΉΣΜ·Ζ¥”ΠΙΐ≥ΧΘ§ΚΆΈο÷ άύ±π≈–ΕœΖ÷ΈωΜ·―ßΦϋΒΡ÷÷άύΘΜ
(4)ΗυΨί”–ΜζΈο÷–ΧΦ‘≠Ή”ΒΡ≥…ΦϋΖΫ ΫΘ§≈–ΕœΩ’ΦδΙΙ–ΆΘ§ΫχΕχ≈–ΕœΧΦ‘≠Ή”‘”Μ·ΖΫ ΫΘΜ
(5)ΔΌ”Π”Ο‘”Μ·ΙλΒάάμ¬έΦΤΥψ÷––Ρ‘≠Ή”ΒΡΦέΒγΉ”Ε‘ ΐ»ΖΕ®‘”Μ·ΖΫ ΫΖ÷Έω»ΖΕ®ΝΔΧεΙΙ–ΆΘΜ
ΔΎΫαΚœΨßΑϊΆΦ ΨΦΤΥψΨßΑϊ÷–Ης‘≠Ή”ΒΡΗω ΐ ι–¥ΤδΖ÷Ή” ΫΘ§‘ΌΫαΚœΨßΑϊΈΔΝΘΒΡœύΜΞΈΜ÷ΟΙΊœΒΦΤΥψΈΔΝΘΒΡΑκΨΕΓΘ
(1) TiΈΣ38Κ≈‘ΣΥΊΘ§ΜυΧ§ν―‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣΘΚ[Ar]3d24s2Θ§‘ρΦέ≤ψΒγΉ”≈≈≤ΦΆΦΈΣ![]() ΘΜΜυΧ§O‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ1s22s22p4Θ§ΉνΗΏΡήΦΕΈΣpΘ§ΤδΒγΉ”‘Τ¬÷άΣΈΣ―ΤΝε–ΆΘΜ
ΘΜΜυΧ§O‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ1s22s22p4Θ§ΉνΗΏΡήΦΕΈΣpΘ§ΤδΒγΉ”‘Τ¬÷άΣΈΣ―ΤΝε–ΆΘΜ
(2) TiCl4ΒΡ»έΓΔΖ–ΒψΖ÷±πΈΣ205KΓΔ409KΘ§”κCCl4ΫαΙΙœύΥΤΘ§ΕΦ τ”ΎΖ÷Ή”ΨßΧεΘ§Ζ÷Ή”ΨßΧεΒΡ»έΖ–Βψ”κΖ÷Ή”ΦδΉς”ΟΝΠ”–ΙΊΘ§œύΕ‘Ζ÷Ή”÷ ΝΩ‘Ϋ¥σΘ§Ζ÷Ή”ΦδΉς”ΟΝΠ‘Ϋ¥σΘ§»έΖ–Βψ‘ΫΗΏΘ§TiCl4ΒΡœύΕ‘Ζ÷Ή”÷ ΝΩ¥σ”ΎCCl4Θ§Ζ÷Ή”ΦδΉς”ΟΝΠΗϋ¥σΘΜ
(3)ΗυΨίΉΣΜ·Ιΐ≥ΧTiCl4Ω…»ή”Ύ≈®―ΈΥαΒΟH2[TiCl6]Θ§Ω…Ω¥Ήω–Έ≥…“Μ÷÷ΥαΘ§Υυ”–ΒΡΥαΕΦ τ”ΎΙ≤ΦέΜ·ΚœΈοΘ§œρ»ή“Κ÷–Φ”»κNH4Cl≈®»ή“ΚΩ…Έω≥ωΜΤ…ΪΒΡ(NH4)2[TiCl6]ΨßΧεΘ§Ω…Ω¥ΉωΥαΗζ―ΈΖ¥”Π…ζ≥…(NH4)2[TiCl6]Θ§≤ζΈο÷–Κ§”–οßΗυάκΉ”Θ§ΗυΨί“‘…œΖ÷ΈωΘ§(NH4)2[TiCl6]ΨßΧε÷–Κ§”–Ι≤ΦέΦϋΚΆάκΉ”ΦϋΘ§Ι ¥πΑΗ―ΓABΘΜ
(4) CH3CH2OHΚΆCH3OCH3÷–ΒΡΧΦ‘≠Ή”ΕΦ «“‘ΒΞΦϋ–Έ Ϋ≥…ΦϋΘ§ΫαΙΙ”κΦΉΆιœύΥΤΘ§ΕΦ «ΥΡΟφΧεΫαΙΙΘ§HCHOΒΡΧΦ‘≠Ή”Κ§”–ΧΦ―θΥΪΦϋΘ§Ζ÷Ή”÷–Υυ”–‘ΎΆ§“ΜΤΫΟφΘ§ΈΣΤΫΟφ»ΐΫ«–ΈΘ§ΗυΨίΙΙ–ΆΩ…÷ΣΘ§»ΐΗωΖ÷Ή”÷–C‘≠Ή”ΒΡVSEPRΡΘ–Ά≤ΜΆ§”ΎΤδΥϊΖ÷Ή”ΒΡ «HCHOΘ§ΗυΨίΙΙ–ΆΩ…ΒΟΘ§ΗΟΖ÷Ή”÷–CΒΡΙλΒά‘”Μ·άύ–ΆΈΣsp2‘”Μ·ΘΜ
(5)ΔΌBaCO3÷–“θάκΉ”ΈΣCO32-Θ§÷––Ρ‘≠Ή”ΈΣΧΦ‘≠Ή”Θ§ΤδΦέ≤ψΒγΉ”Ε‘ ΐ=3+![]() =3Θ§ΧΦ‘≠Ή”ΈΣsp2‘”Μ·Θ§ΗΟ“θάκΉ””…4Ηω‘≠Ή”ΙΙ≥…Θ§‘ρΝΔΧεΙΙ–ΆΈΣΤΫΟφ»ΐΫ«–ΈΘΜ
=3Θ§ΧΦ‘≠Ή”ΈΣsp2‘”Μ·Θ§ΗΟ“θάκΉ””…4Ηω‘≠Ή”ΙΙ≥…Θ§‘ρΝΔΧεΙΙ–ΆΈΣΤΫΟφ»ΐΫ«–ΈΘΜ
ΔΎΗυΨίΨßΑϊΆΦ ΨΘ§TiΈΜ”ΎΨßΑϊΒΡΕΞΒψΘ§TiΒΡ ΐΡΩ=8ΓΝ![]() =1Θ§Ba‘≠Ή”ΈΜ”ΎΨßΑϊΒΡΡΎ≤ΩΘ§ ΐΡΩΈΣ1Θ§Ζ÷ΈωΦΤΥψΖ÷Ή” ΫΚΆΝΘΉ”ΑκΨΕΘΜO‘≠Ή”ΈΜ”ΎΨßΑϊΒΡάβ…œΘ§Τδ ΐΡΩ=12ΓΝ
=1Θ§Ba‘≠Ή”ΈΜ”ΎΨßΑϊΒΡΡΎ≤ΩΘ§ ΐΡΩΈΣ1Θ§Ζ÷ΈωΦΤΥψΖ÷Ή” ΫΚΆΝΘΉ”ΑκΨΕΘΜO‘≠Ή”ΈΜ”ΎΨßΑϊΒΡάβ…œΘ§Τδ ΐΡΩ=12ΓΝ![]() =3Θ§‘ρ‘ρν―Υα±ΒΒΡΜ·―ß ΫΈΣBaTiO3ΘΜ“―÷ΣΨßΑϊ±Ώ≥ΛΈΣa pmΘ§O2Θ≠ΒΡΑκΨΕΈΣbpmΘ§ΗυΨίΆΦ ΨΘ§ΨßΑϊ±Ώ≥Λ= 2r(Ti4+)+2r(O2-)=apmΘ§‘ρr(Ti4+)=
=3Θ§‘ρ‘ρν―Υα±ΒΒΡΜ·―ß ΫΈΣBaTiO3ΘΜ“―÷ΣΨßΑϊ±Ώ≥ΛΈΣa pmΘ§O2Θ≠ΒΡΑκΨΕΈΣbpmΘ§ΗυΨίΆΦ ΨΘ§ΨßΑϊ±Ώ≥Λ= 2r(Ti4+)+2r(O2-)=apmΘ§‘ρr(Ti4+)=![]() pmΘΜΨßΑϊΟφΕ‘Ϋ«œΏΒΡ≥ΛΕ»=2r(O2-)+2r(Ba2+)=
pmΘΜΨßΑϊΟφΕ‘Ϋ«œΏΒΡ≥ΛΕ»=2r(O2-)+2r(Ba2+)=![]() a pmΘ§r(Ba2+)=
a pmΘ§r(Ba2+)=![]() pmΓΘ
pmΓΘ

 ΤΏ–«ΆΦ ιΩΎΥψΥΌΥψΧλΧλΝΖœΒΝ–¥πΑΗ
ΤΏ–«ΆΦ ιΩΎΥψΥΌΥψΧλΧλΝΖœΒΝ–¥πΑΗ ≥θ÷–―ß“ΒΩΦ ‘ΒΦ”κΝΖœΒΝ–¥πΑΗ
≥θ÷–―ß“ΒΩΦ ‘ΒΦ”κΝΖœΒΝ–¥πΑΗ