��Ŀ����
����Ŀ��
AlN ����������Ҫ�İ뵼����ϣ�Ga(��)��P��As(��)�����γɻ�����뵼����ϵ���ҪԪ�ء��ش��������⣺
(1)As��̬ԭ�ӵĵ���ռ����______���ܲ㣬����ܼ��ĵ����Ų�ʽΪ______����Asλ��ͬһ���ڣ���δ�ɶԵ�����Ҳ��ͬ��Ԫ�ػ���______�֡�
(2)Ԫ�����ڱ��У���P���ڵ�4��Ԫ���е縺��������______ (��Ԫ������)��Si��P��S����Ԫ�ص� ��һ�������ɴ�С��˳����______��
(3)NH3��PH3��AsH3���ߵķе��ɸߵ��͵�˳����______��ԭ����______��
(4)��������P4�����γɵķ��Ӿ��壬P4���ӳ���������ṹ��Pԭ��λ������������ĸ����㣬��Pԭ�ӵ��ӻ���ʽΪ_____������������CS2��������ˮ��ԭ����__________________��
(5)����GaxIn1-xAs(������)�Ȳ��ϣ������̫���ܵ�ص�Ч�ʡ�GaxIn1-xAs�������ξ�����ÿ����������Ķ���һ��ԭ�ӣ������ڲ���4 ��ԭ�ӣ���þ����к���_________����ԭ�ӡ�
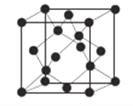
(6)AlN����ľ����ṹ����ʯ����(����ͼ)���辧���ı߳�Ϊ�� pm��NA��ʾ�����ӵ���������þ�����ܶ�Ϊ__________g��cm-3��
���𰸡� 4 4p3 2 N P>S>Si NH3>AsH3>PH3 NH3���Ӵ����������е��AsH3��PH3�ĸߣ�AsH3��PH3���Ӽ䲻���������AsH3����Է����������Ӽ�����������е��PH3�ĸ� sp3 P4��CS2���ǷǼ��Է��ӣ�H2O�Ǽ��Է��ӣ�������������ԭ����P4������CS2��������ˮ 4 ![]()
����������1����������Ų�ʽ����д���ܼ������ߵ͵��жϵȣ�Asλ�ڵ�������VA�壬�����Ų�ʽΪ1s22s22p63s23p63d104s24p3������ռ����4���ܲ㣬����ܼ���4p���������ܼ��ĵ����Ų�ʽΪ4p3��Asδ�ɶԵ�����Ϊ3����˵�������δ�ɶԵ�����Ϊ3��Ԫ����V��Co����2��Ԫ�أ���2������縺�Թ��ɡ���һ�����ܵĹ��ɣ�P�����ڵ�4��Ԫ�طֱ���Si��S��N��As��ͬ���ڴ������ҵ縺������ͬ������ϵ��µ縺�Լ�С��N�ĵ縺�Դ���S��������ڵ�4��Ԫ�ص縺��������N��ͬ���ڴ������ҵ�һ����������IIA>IIIA��VA>VIA����˵�һ�����ܵĴ�С˳����P>S>Si����3�������۷е�ߵ͵��жϣ�N��P��As����ͬһ���壬NH3���Ӽ���ڷ��Ӽ���������������������Ӽ���������NH3�ķе�����������ߣ�AsH3����Է�����������PH3��AsH3�ķ��Ӽ�����������PH3��AsH3�ķе����PH3������С˳����NH3>AsH3>PH3����4�������ӻ������жϡ��ܽ��ԣ�P4�ĽṹʽΪ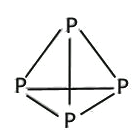 ��P��3��������1���µ��Ӷԣ�����ӻ�����Ϊsp3��CS2Ϊ�Ǽ��Է��ӣ�P4Ϊ�Ǽ��Է��ӣ�H2OΪ���Է��ӣ������������ܣ�����������CS2��������ˮ����5��ÿһ����������Ķ���һ��ԭ�ӣ������ڲ���4��ԭ�ӣ���������ԭ������Ϊ8��1/8��6��1/2��4=8,�ɻ�ѧʽ��֪������As��Ŀռԭ��������1/2����������As��ԭ����ĿΪ8��1/2=4����6�����龧���ļ��㣬����AlN�ľ����Ľṹ���þ���������Ϊ
��P��3��������1���µ��Ӷԣ�����ӻ�����Ϊsp3��CS2Ϊ�Ǽ��Է��ӣ�P4Ϊ�Ǽ��Է��ӣ�H2OΪ���Է��ӣ������������ܣ�����������CS2��������ˮ����5��ÿһ����������Ķ���һ��ԭ�ӣ������ڲ���4��ԭ�ӣ���������ԭ������Ϊ8��1/8��6��1/2��4=8,�ɻ�ѧʽ��֪������As��Ŀռԭ��������1/2����������As��ԭ����ĿΪ8��1/2=4����6�����龧���ļ��㣬����AlN�ľ����Ľṹ���þ���������Ϊ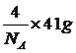 �����������Ϊ(a��10��10)3cm3�������ܶȵĶ��壬�ܶ�Ϊ
�����������Ϊ(a��10��10)3cm3�������ܶȵĶ��壬�ܶ�Ϊ![]() g/cm3��
g/cm3��

����Ŀ���������ڵ�Cr��Fe��Co��Ni��Cu��Zn������������γ�����������������鼰���ǵĻ�����㷺Ӧ���ڳ�������ϵ�������ش��������⣺
��1��Fe2+�ĺ�������Ų�ʽΪ_________________��
��2��NH3��һ�ֺܺõ����壬NH3�ķе�______(����������������������������)AsH3��
��3����ѧ��ͨ��X���߲�õ����ṹʾ��ͼ�ɼ�ʾ������ͼ�����߱�ʾ��������Ϊ________________��

��4������������CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4�����������幹�͡�Ni(CO)4������________(����)��
A��ˮ����B�����Ȼ�̼ C���� D����������Һ
��5��As��±������۵�������
AsCl3 | AsBr3 | AsI3 | |
�۵�/K | 256.8 | 304 | 413 |
����±�����۵�����ԭ����________________��
��6����FeCl3��Һ�е���EDTA�Լ��ɵ������A,��ṹ��ͼ��ʾ��ͼ��M����Fe3+����Fe3+�뵪ԭ��֮���γɵĻ�ѧ����_________��Fe3+����λ��Ϊ_________��

����Ŀ���屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£������ºϳɲ���ش����⣺
��֪��C6H6������+Br2���壩��C6H5Br���屽��+HBr������Ҫ��������������
Һ�塢�廯��������������Ʒ�Ӧ�������屽�����������Ʒ�Ӧ���廯��������ˮ��

�� | �� | �屽 | |
�ܶ�/g��cm-3 | 0.88 | 3.10 | 1.50 |
�е�/�� | 80 | 59 | 156 |
ˮ���ܽ�� | �� | �� | �� |
��1������c������_________��
��2����a�м���15 mL��ˮ����������м����b��С�ļ���4.0 mL Һ̬�塣��a�е��뼸���壬�а�������������Ϊ������__________���壬�����μ���Һ����ꡣװ��d��������____________________��
��3��Һ�����������в�������ᴿ��
����a�м���10 mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10 mLˮ��8 mL10����NaOH��Һ��10 mLˮϴ�ӡ�NaOH��Һϴ�ӵ�������_____________________���ڶ���ˮϴ��Ŀ��Ϊ________________��
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˡ������Ȼ��Ƶ�Ŀ����______��
��4��������������������屽�л����в������ʣ�Ҫ��һ���ᴿ��ѡ���װ��Ϊ___��������ȷѡ��ǰ����ĸ����

��5���ڸ�ʵ���У�a���ݻ����ʺϵ���________��������ȷѡ��ǰ����ĸ����
A��25 mL B��50 mL C��250 mL D��500 mL



