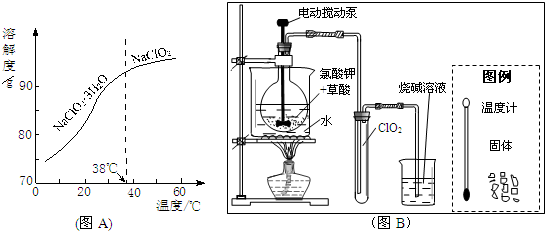
��Ŀ����
ͭ��Ũ�����м����ܷ������·�Ӧ��Cu+2H2SO4
CuSO4+SO2��+2H2O��ij��ѧ��ȤС���ͬѧ�������ͼ��ʾʵ�飬��̽��ͭ��Ũ���ᷴӦ�����������������ʣ�����Ҳһ�����̽������������µ����⣮
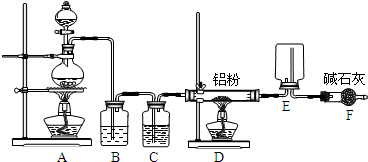
��1��ͼ�б�ŵ��������Ʒֱ�Ϊ��a______��b______��
��2����ʵ�鿪ʼ���װ�ó��ֵ������������жϣ�
�ٵ�A�й۲쵽ͭƬ�ܽ⡢����������ʱ�����ܹ۲쵽��Һ��______ɫ������
��B�е�ʯ����Һ����______ɫ��˵���������е�SO2��һ�����Ե����壻
��C��Ʒ����Һ�ɺ�ɫ�����ɫ��˵���������е�SO2����______�ԣ�
��3��C���ݳ���SO2���徭װ��D��ʢװ��______��Һ�����ͨ��װ��E���ô����ǵ�ľ�����G�У�ľ���ܸ�ȼ��˵��E�еķ�Ӧ��______���ɣ�E�з�Ӧ�Ļ�ѧ����ʽ�ǣ�______��
��4��Fװ�õ������ǣ�______��
| ||

��1��ͼ�б�ŵ��������Ʒֱ�Ϊ��a______��b______��
��2����ʵ�鿪ʼ���װ�ó��ֵ������������жϣ�
�ٵ�A�й۲쵽ͭƬ�ܽ⡢����������ʱ�����ܹ۲쵽��Һ��______ɫ������
��B�е�ʯ����Һ����______ɫ��˵���������е�SO2��һ�����Ե����壻
��C��Ʒ����Һ�ɺ�ɫ�����ɫ��˵���������е�SO2����______�ԣ�
��3��C���ݳ���SO2���徭װ��D��ʢװ��______��Һ�����ͨ��װ��E���ô����ǵ�ľ�����G�У�ľ���ܸ�ȼ��˵��E�еķ�Ӧ��______���ɣ�E�з�Ӧ�Ļ�ѧ����ʽ�ǣ�______��
��4��Fװ�õ������ǣ�______��
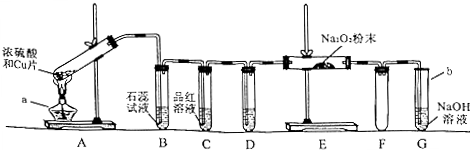
��1��װ��ͼ������֪aΪ�ƾ��ƣ�bΪ�Թܣ�
�ʴ�Ϊ���ƾ��ƣ��Թܣ�
��2����Ũ�����ͭ���ȷ�Ӧ��������ͭ��Һ����ɫ����A�й۲쵽ͭƬ�ܽ⡢����������ʱ�����ܹ۲쵽��Һ������
�ʴ�Ϊ������
��ͭ��Ũ���ᷴӦ���ɶ�����������ͨ��ʯ����Һ�ж��������ˮ��Ӧ����������������ԣ�ʹʯ����Һ���ɫ��˵�������������������壬
�ʴ�Ϊ���죻
�۶����������Ư���ԣ�ͨ��Ʒ����Һ��ʹ��Һ��ɫ��
�ʴ�Ϊ��Ư�ף�
��3��C���ݳ���SO2���徭װ��D�е�Ũ��������ͨ��װ��E��������ʹ�����ǵ�ľ����ȼ������Ϊ���������ݶ�����̼�������Ʒ�Ӧ�IJ������д����������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2SO2=2Na2SO3+O2��
�ʴ�Ϊ��Ũ���ᣬ������2Na2O2+2SO2=2Na2SO3+O2��
��4��Fװ���ǰ�ȫװ�ã����Է�ֹG����Һ������Eװ��ը�Ѳ����ܣ�
�ʴ�Ϊ����ֹ��������ʱG����Һ����װ��E�У�
�ʴ�Ϊ���ƾ��ƣ��Թܣ�
��2����Ũ�����ͭ���ȷ�Ӧ��������ͭ��Һ����ɫ����A�й۲쵽ͭƬ�ܽ⡢����������ʱ�����ܹ۲쵽��Һ������
�ʴ�Ϊ������
��ͭ��Ũ���ᷴӦ���ɶ�����������ͨ��ʯ����Һ�ж��������ˮ��Ӧ����������������ԣ�ʹʯ����Һ���ɫ��˵�������������������壬
�ʴ�Ϊ���죻
�۶����������Ư���ԣ�ͨ��Ʒ����Һ��ʹ��Һ��ɫ��
�ʴ�Ϊ��Ư�ף�
��3��C���ݳ���SO2���徭װ��D�е�Ũ��������ͨ��װ��E��������ʹ�����ǵ�ľ����ȼ������Ϊ���������ݶ�����̼�������Ʒ�Ӧ�IJ������д����������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2SO2=2Na2SO3+O2��
�ʴ�Ϊ��Ũ���ᣬ������2Na2O2+2SO2=2Na2SO3+O2��
��4��Fװ���ǰ�ȫװ�ã����Է�ֹG����Һ������Eװ��ը�Ѳ����ܣ�
�ʴ�Ϊ����ֹ��������ʱG����Һ����װ��E�У�

��ϰ��ϵ�д�
�����Ŀ