��Ŀ����
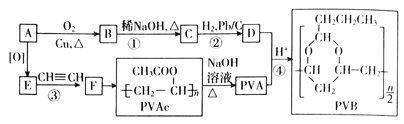
����Ŀ���ϳɾ����������オ���Ե��л��߷��Ӳ������л���ѧ�о�����Ҫ����֮һ���۴�����ϩ����PVAc��ˮ�����ɵľ���ϩ����PVA���������������オ���ԣ�������������ȫ�����в����PVB���йغϳ�·����ͼ�����ݷ�Ӧ�����Ͳ�����ȥ����
��֪��I.AΪ����һԪ��������������������ԼΪ34.8%��
II.![]()
![]() ��
��
III.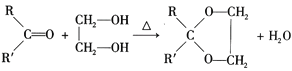 (R��R���ɱ�ʾ��������ԭ��)
(R��R���ɱ�ʾ��������ԭ��)
��ش�
��1��C�й����ŵ�����Ϊ_____________��д��C�ķ�ʽ�칹��Ľṹ��ʽ��_______________���÷����������______________��ԭ�ӹ���
��2��D�뱽��ȩ��Ӧ�Ļ�ѧ����ʽΪ_________________________________________________��
��3���۵ķ�Ӧ������_________________________
��4��д��������F������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ_____________________________��
��5������������Ϣ����ƺϳ�·�ߣ���������Ϊԭ�ϣ��������Լ���ѡ���ϳ�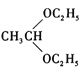 ��_____________
��_____________
���𰸡�̼̼˫����ȩ��  9 CH3CH2CH2CHO+
9 CH3CH2CH2CHO+![]()
![]()
![]() +H2O �ӳɷ�Ӧ HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC(CH3)=CH2(��д����) CH3CH2Br
+H2O �ӳɷ�Ӧ HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC(CH3)=CH2(��д����) CH3CH2Br![]() CH3CH2OH
CH3CH2OH![]() CH3CHO
CH3CHO![]()

��������
AΪ����һԪ����ͨʽΪCnH2n+2O����������������ԼΪ34.8%������![]() ��100%=34.8%�����n=2����AΪCH3CH2OH��A��������EΪCH3COOH��E����Ȳ�����ӳɷ�Ӧ����FΪCH3COOCH=CH2��F�����Ӿ۷�Ӧ�õ�PVAcΪ
��100%=34.8%�����n=2����AΪCH3CH2OH��A��������EΪCH3COOH��E����Ȳ�����ӳɷ�Ӧ����FΪCH3COOCH=CH2��F�����Ӿ۷�Ӧ�õ�PVAcΪ![]() ������ˮ��õ�PVA(
������ˮ��õ�PVA(![]() )��A��ͭ�������������������õ�BΪCH3CHO��B������Ϣ���еķ�Ӧ�õ�CΪCH3CH=CHCHO��C������ԭ��Ӧ����DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB��
)��A��ͭ�������������������õ�BΪCH3CHO��B������Ϣ���еķ�Ӧ�õ�CΪCH3CH=CHCHO��C������ԭ��Ӧ����DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB��
(1)CΪCH3CH=CHCHO��C�й����ŵ�������̼̼˫����ȩ����C�ķ�ʽ�칹��Ľṹ��ʽΪ ����ת̼̼��������ʹ̼̼˫��ƽ����-CHOƽ�湲�棬����ʹ����1��Hԭ�Ӵ���ƽ���ڣ��÷����������9��ԭ�ӹ�ƽ�棬�ʴ�Ϊ̼̼˫����ȩ����
����ת̼̼��������ʹ̼̼˫��ƽ����-CHOƽ�湲�棬����ʹ����1��Hԭ�Ӵ���ƽ���ڣ��÷����������9��ԭ�ӹ�ƽ�棬�ʴ�Ϊ̼̼˫����ȩ���� ��9��
��9��
(2)D�뱽��ȩ��Ӧ�Ļ�ѧ����ʽΪ��CH3CH2CH2CHO+![]()
![]()
![]() +H2O���ʴ�ΪCH3CH2CH2CHO+
+H2O���ʴ�ΪCH3CH2CH2CHO+![]()
![]()
![]() +H2O��
+H2O��
(3)��Ӧ����CH3COOH����Ȳ�����ӳɷ�Ӧ��CH3COOCH=CH2���ʴ�Ϊ�ӳɷ�Ӧ��
(4)FΪCH3COOCH=CH2����F������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪ��HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC(CH3)=CH2���ʴ�ΪHCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC(CH3)=CH2(��д����)
(5)�����鷢��ˮ�ⷴӦ�����Ҵ����Ҵ�����������������ȩ����ȩ���Ҵ���Ӧ�õ� ���ϳ�·������ͼΪ��CH3CH2Br
���ϳ�·������ͼΪ��CH3CH2Br![]() CH3CH2OH
CH3CH2OH![]() CH3CHO
CH3CHO![]()
 ���ʴ�ΪCH3CH2Br
���ʴ�ΪCH3CH2Br![]() CH3CH2OH
CH3CH2OH![]() CH3CHO
CH3CHO![]()
 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ǿ��������dz���Ҫ��һ�����ӷ�Ӧ����֪��
Ka1 | Ka2 | |
H2SO3 |
|
|
H2CO3 |
|
|
�������ӷ�Ӧ����ȷ���У���������
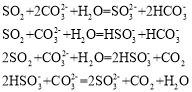
A.0��B.1��C.2��D.3��