��Ŀ����
��λ����һ������Ĺ��ۼ��������õ��Ӷ���ijԭ�ӵ������ṩ����һȱ���ӵ����ӽ�ϡ���NH4��������NH3����ԭ���ṩ���Ӷԣ���H����ȱ���ӣ�ͨ����λ���γɵġ��ݴˣ��ش��������⣺
��1�����������п��ܴ�����λ������________��
A��CO2 B��H3O�� C��CH4 D��H2SO4
��2�����ᣨH3BO3����Һ�����ԣ���д������뷽��ʽ��___________________��
��3����ѧ�Ҷ�H2O2�ṹ����ʶ�����˽�Ϊ�����Ĺ��̣��������ѧ��������ֹ۵㣺
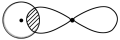
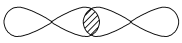
�ף�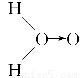 ���ң�H��O��O��H��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѡ���ѧ��Baeyer��VilliyerΪ�о�H2O2�Ľṹ����Ʋ����������ʵ�飺a.��C2H5OH��ŨH2SO4��Ӧ���ɣ�C2H5��2SO4��ˮ��b.���Ƶõģ�C2H5��2SO4��H2O2��Ӧ��ֻ����A��H2SO4��c.�����ɵ�A��H2��Ӧ����֪�÷�Ӧ��H2����ԭ������
���ң�H��O��O��H��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѡ���ѧ��Baeyer��VilliyerΪ�о�H2O2�Ľṹ����Ʋ����������ʵ�飺a.��C2H5OH��ŨH2SO4��Ӧ���ɣ�C2H5��2SO4��ˮ��b.���Ƶõģ�C2H5��2SO4��H2O2��Ӧ��ֻ����A��H2SO4��c.�����ɵ�A��H2��Ӧ����֪�÷�Ӧ��H2����ԭ������
�����H2O2�Ľṹ�����ʾ��ʵ��c�л�ѧ��Ӧ����ʽΪ��Aд�ṹ��ʽ��_____________________________________________________________��
��Ϊ�˽�һ��ȷ��H2O2�Ľṹ������Ҫ��ʵ��c������һ��ʵ��d�������d��ʵ�鷽����____________________________________________________________��
��1��BD
��2��H3BO3��H2O H����[B��OH��4]��
H����[B��OH��4]��
��3�� 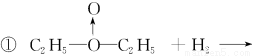
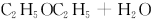
������ˮ����ͭ����c�ķ�Ӧ��������û��ˮ�������������𰸣�
�������������ۺϿ�����λ�����γɺ����������ʡ�����ʱҪע����λ���γ������е�һ���ṩ�¶Ե��ӣ���һ���ṩ�չ����
��1����������Ϣ�ɵ������ۣ����ܸ���H����������һ�㺬����λ����
��2����ԭ��Ϊȱ����ԭ�ӣ�H3BO3�ĵ���ʵ����Bԭ�Ӻ�ˮ�е�OH���γ���λ����ˮ������H�����ֳ����ԡ�
��3��������������λ�������ʵķ�Ӧ�ص������

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д��±���ijЩԭ�Ӿ�����۵��Ӳ��
ԭ�Ӿ��� | ���ʯ | ������ | ̼���� | ʯӢ | �� | �� |
�۵�/�� | 3 900 | 3 000 | 2 700 | 1 710 | 1 410 | 1 211 |
Ӳ�� | 10 | 9.5 | 9.5 | 7 | 6.5 | 6.0 |
�������е����ݣ��ж�����������ȷ���ǣ� ����
������ԭ�Ӿ����ԭ������Խ�࣬������۵�Խ��
������ԭ�Ӿ����ԭ�Ӽ�Ĺ��ۼ�����Խ������۵�Խ��
������ԭ�Ӿ����ԭ�ӵİ뾶Խ�����Ӳ��Խ��
������ԭ�Ӿ����ԭ�ӵİ뾶ԽС�������Ӳ��Խ��
A���٢� B���ۢ� C���٢� D���ڢ�