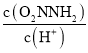ЬтФПФкШн
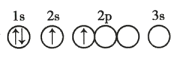
ЁОЬтФПЁПСзЫсТШрЪЧвЛжжЙХРЯЕФПЙХБМВКЭжЮСЦАЂУзАЭВЁвЉЮяжжКЭЮяЃЌвВЪЧвЛжжжЮСЦздЩэУтвпадМВВЁЕФвЉЮяЁЃ2020Фъ2дТ19ШедкЁЖаТаЭЙкзДВЁЖОЗЮбзеяСЦЗНАИ(ЪдааЕкСљАц)ЁЗРяУцЬсГіаТдіПкЗўСзЫсТШдћзїЮЊПЙаТЙкВЁЖОИаШОжЮСЦгУвЉЁЃЦфжаМфЬхHЕФвЛжжКЯГЩТЗЯпШчЯТЃК
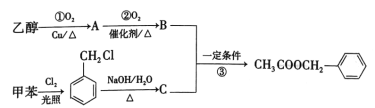
вбжЊЃКAЮЊАыЯЫЮЌЫиЕФвЛжжЃЌЪєгкЖрЬЧЃЌBЮЊЮьЬЧЃЌ BЁЂCжаОљКЌгаШЉЛљЃЛCжаКЌгаЮхдЊЛЗзДНсЙЙЧвКЌвЛжжгыDЯрЭЌЕФЙйФмЭХЃЛCжаВЛКЌЬМЬМШ§МќЁЃ
(1)DжаЫљКЌЙйФмЭХЕФУћГЦЪЧ______ЁЃ
(2)ЂйЕФЗДгІЪдМСКЭЬѕМўЪЧ________ЁЃ
(3)ЬМдзгЩЯСЌга4ИіВЛЭЌЕФдзгЛђЛљЭХЪБЃЌИУЬМГЦЮЊЪжадЬМЃЌаДГіBЕФНсЙЙМђЪНЃЌгУаЧКХ (*)БъГіBжаЕФЪжадЬМдзг______ЁЃ
(4)CЕФНсЙЙМђЪНЮЊ_______ЁЃ
(5)ЂнЕФЗДгІРраЭЪЧ________ЃЌЂоЕФЛЏбЇЗНГЬЪНЮЊ________ЁЃ
(6)ЛЏКЯЮяWЪЧEЕФЭЌЗжвьЙЙЬхЃЌWФмЗЂЩњЫЎНтЗДгІЃЌЗћКЯЬтвтЕФWга_____жж(ВЛКЌСЂЬхНсЙЙ)ЃЌКЫДХЙВеёЧтЦзжЛгаСНзщЗхЕФНсЙЙМђЪНЪЧ________ЁЃ
(7)ЩшМЦвд![]() (ЛЗбѕБћЭщ)ЮЊдСЯжЦБИ
(ЛЗбѕБћЭщ)ЮЊдСЯжЦБИ![]() ЕФКЯГЩТЗЯп(ЮоЛњЪдМСШЮгУ)______ЁЃ
ЕФКЯГЩТЗЯп(ЮоЛњЪдМСШЮгУ)______ЁЃ
ЁОД№АИЁПУбМќ H2O/H+ЃЌМгШШ 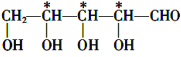
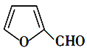 ШЁДњЗДгІ
ШЁДњЗДгІ ![]() +NH(C2H5)2Ёњ
+NH(C2H5)2Ёњ![]() +HCl 9 HCOOC(CH3)3
+HCl 9 HCOOC(CH3)3 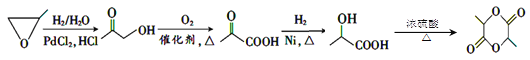 Лђ
Лђ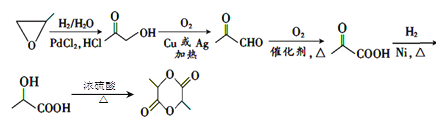
ЁОНтЮіЁП
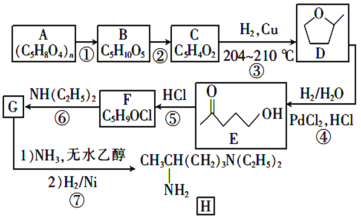
AЗжзгЪНЪЧ(C5H8O4)nЃЌЮЊАыЯЫЮЌЫиЕФвЛжжЃЌдкЫсадЬѕМўЯТЃЌдкМгШШЪБЗЂЩњЫЎНтЗДгІВњЩњBЮЊЮьЬЧЃЌЧвКЌгаШЉЛљЃЌдђBНсЙЙМђЪНЮЊCH2OH(CHOH)3CHOЃЌBдквЛЖЈЬѕМўЯТЗДгІВњЩњC5H4O2ЃЌИљОнBЁЂCЗжзгЪНЕФВЛЭЌЃЌПЩжЊCЗжзгБШBЩй3ИіH2OЕФзщГЩЃЌЧвКЌгаШЉЛљЃЌКЌгаЮхдЊЛЗзДНсЙЙЃЌЧвCжаВЛКЌЬМЬМШ§МќЃЌЫЕУїBБфЮЊCЗЂЩњСЫЯћШЅЗДгІЃЌдђCНсЙЙМђЪНЮЊ ЃЛCгыH2дкCuДцдкКЭМгШШ204ЁЋ210ЁцЪБЃЌЗДгІВњЩњDЃК
ЃЛCгыH2дкCuДцдкКЭМгШШ204ЁЋ210ЁцЪБЃЌЗДгІВњЩњDЃК![]() ЃЌDдквЛЖЈЬѕМўЯТЗДгІВњЩњEЃК
ЃЌDдквЛЖЈЬѕМўЯТЗДгІВњЩњEЃК![]() ЃЌEгыHClЗЂЩњШЁДњЗДгІВњЩњFЃК
ЃЌEгыHClЗЂЩњШЁДњЗДгІВњЩњFЃК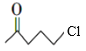 ЃЌ
ЃЌ гыNH(C2H5)2ЗЂЩњШЁДњЗДгІВњЩњGЃК
гыNH(C2H5)2ЗЂЩњШЁДњЗДгІВњЩњGЃК![]() ЃЌGгыH2ЯШЗЂЩњМгГЩЗДгІЃЌШЛКѓдйгыNH3ЗЂЩњШЁДњЗДгІВњЩњHЃК
ЃЌGгыH2ЯШЗЂЩњМгГЩЗДгІЃЌШЛКѓдйгыNH3ЗЂЩњШЁДњЗДгІВњЩњHЃК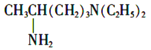 ЁЃ
ЁЃ
ИљОнЩЯЪіЗжЮіПЩжЊBЪЧCH2OH(CHOH)3CHOЃЌCЪЧ ЃЌFЪЧ
ЃЌFЪЧ ЃЌGЪЧ
ЃЌGЪЧ![]() ЁЃ
ЁЃ
(1)DНсЙЙМђЪНЪЧ![]() ЃЌКЌгаЕФЙйФмЭХЮЊУбМќЃЛ
ЃЌКЌгаЕФЙйФмЭХЮЊУбМќЃЛ
(2)AЪЧвЛжжАыЯЫЮЌЫиЃЌдкЫсзїДпЛЏМСЬѕМўЯТМгШШЃЌЗЂЩњЫЎНтЗДгІВњЩњЮьЬЧBЃКCH2OH(CHOH)3CHOЃЌЫљвдЂйЕФЗДгІЪдМСКЭЬѕМўЪЧH2O/H+ЃЌМгШШЃЛ
(3) BНсЙЙМђЪНЪЧЃКCH2OH(CHOH)3CHOЃЌИљОнЪжадЬМдзгЕФКЌвхЃЌгУаЧКХ (*)БъГіBжаЕФЪжадЬМдзгЮЊЃК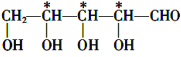 ЃЛ
ЃЛ
(4)CЕФНсЙЙМђЪНЮЊ ЃЛ
ЃЛ
(5)EЪЧ![]() ЃЌгыHClдквЛЖЈЬѕМўЯТЗЂЩњШЁДњЗДгІВњЩњFЃК
ЃЌгыHClдквЛЖЈЬѕМўЯТЗЂЩњШЁДњЗДгІВњЩњFЃК ЃЌЫљвдЂнЕФЗДгІРраЭЪЧШЁДњЗДгІЃЛFЪЧ
ЃЌЫљвдЂнЕФЗДгІРраЭЪЧШЁДњЗДгІЃЛFЪЧ ЃЌгыNH(C2H5)2дквЛЖЈЬѕМўЯТЗЂЩњШЁДњЗДгІВњЩњG:
ЃЌгыNH(C2H5)2дквЛЖЈЬѕМўЯТЗЂЩњШЁДњЗДгІВњЩњG: ![]() ЃЌЫљвдЂоЕФЛЏбЇЗНГЬЪНЮЊ
ЃЌЫљвдЂоЕФЛЏбЇЗНГЬЪНЮЊ![]() +NH(C2H5)2Ёњ
+NH(C2H5)2Ёњ![]() +HClЃЛ
+HClЃЛ
(6)EЪЧ![]() ЃЌЛЏКЯЮяWЪЧEЕФЭЌЗжвьЙЙЬхЃЌWФмЗЂЩњЫЎНтЗДгІЃЌЫЕУїКЌгаѕЅЛљЃЌЪєгкБЅКЭвЛдЊѕЅЃЌПЩФмЪЧHCOO-C4H9ЃЌCH3COOC3H7ЁЂCH3CH2COOC2H5ЁЂC3H7-COOCH3ЃЌгЩгкБћЛљЁЊC3H7га2жжВЛЭЌНсЙЙЃЌЖЁЛљЁЊC4H9гаЫФжжВЛЭЌНсЙЙЃЌЫљвдHCOO-C4H9га4жжНсЙЙЃЛCH3COOC3H7ЁЂC3H7-COOCH3га2жжНсЙЙЃЛCH3CH2COOC2H5жЛга1жжНсЙЙЃЌвђДЫЗћКЯЬтвтЕФWПЩФмжжРрЪ§ФПЮЊ4+2+1+2=9жжЃЌЦфжаКЫДХЙВеёЧтЦзжЛгаСНзщЗхЕФНсЙЙМђЪНЪЧHCOOC(CH3)3ЃЛ
ЃЌЛЏКЯЮяWЪЧEЕФЭЌЗжвьЙЙЬхЃЌWФмЗЂЩњЫЎНтЗДгІЃЌЫЕУїКЌгаѕЅЛљЃЌЪєгкБЅКЭвЛдЊѕЅЃЌПЩФмЪЧHCOO-C4H9ЃЌCH3COOC3H7ЁЂCH3CH2COOC2H5ЁЂC3H7-COOCH3ЃЌгЩгкБћЛљЁЊC3H7га2жжВЛЭЌНсЙЙЃЌЖЁЛљЁЊC4H9гаЫФжжВЛЭЌНсЙЙЃЌЫљвдHCOO-C4H9га4жжНсЙЙЃЛCH3COOC3H7ЁЂC3H7-COOCH3га2жжНсЙЙЃЛCH3CH2COOC2H5жЛга1жжНсЙЙЃЌвђДЫЗћКЯЬтвтЕФWПЩФмжжРрЪ§ФПЮЊ4+2+1+2=9жжЃЌЦфжаКЫДХЙВеёЧтЦзжЛгаСНзщЗхЕФНсЙЙМђЪНЪЧHCOOC(CH3)3ЃЛ
(7)ЛЗбѕБћЭщ(![]() )дкH2/H2OМАPdCl2ЃЌHClзїгУЯТЗДгІВњЩњ
)дкH2/H2OМАPdCl2ЃЌHClзїгУЯТЗДгІВњЩњ![]() ЃЌ
ЃЌ![]() БЛДпЛЏбѕЛЏВњЩњ
БЛДпЛЏбѕЛЏВњЩњ![]() ЃЌИУЮяжЪгыH2ЗЂЩњМгГЩЗДгІВњЩњ
ЃЌИУЮяжЪгыH2ЗЂЩњМгГЩЗДгІВњЩњ![]() ЃЌ2ИіЗжзгЕФ
ЃЌ2ИіЗжзгЕФ![]() гыХЈСђЫсЛьКЯМгШШЃЌЗЂЩњѕЅЛЏЗДгІВњЩњ
гыХЈСђЫсЛьКЯМгШШЃЌЗЂЩњѕЅЛЏЗДгІВњЩњ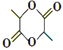 ЃЌЙЪгЩ
ЃЌЙЪгЩ![]() жЦШЁ
жЦШЁ ЕФЗДгІСїГЬЮЊЃК
ЕФЗДгІСїГЬЮЊЃК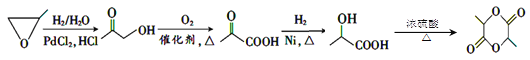 ЃЛЛђЮЊЃК
ЃЛЛђЮЊЃК

 ЪРМЭАйЭЈжїЬхПЮЬУаЁбЇПЮЪБЭЌВНДяБъЯЕСаД№АИ
ЪРМЭАйЭЈжїЬхПЮЬУаЁбЇПЮЪБЭЌВНДяБъЯЕСаД№АИ ЪРМЭАйЭЈгХСЗВтЯЕСаД№АИ
ЪРМЭАйЭЈгХСЗВтЯЕСаД№АИЁОЬтФПЁПЯТСаЖдвЛаЉЪЕбщЪТЪЕКЭРэТлНтЪЭе§ШЗЕФЪЧ
бЁЯю | ЪЕбщЪТЪЕ | РэТлНтЪЭ |
A | HClЦјЬхШмгкЫЎЃЌФмЕМЕч | HClЮЊРызгЛЏКЯЮя |
B | HBrЕФЫсадЧПгкHClЕФЫсад | BrЕФЗЧН№ЪєадБШClЧП |
C |
|
|
D | HFЕФЗаЕуИпгкHCl | FЕФЗЧН№ЪєадБШClЧП |
A.AB.BC.CD.D