��Ŀ����
����Ŀ���±���Ԫ�����ڱ������ڵ�һ���֣�

�밴Ҫ���û�ѧ�����ش��������⣺
(1)Ԫ�آܡ��ޡ�������Ӱ뾶�ɴ�С��˳��Ϊ________________��
(2)Ԫ�آڡ��ۡ��ܵļ��⻯��ķе��ɵ͵��ߵ�˳�� _____________________��
(3)�õ���ʽ��ʾԪ�آ���Ԫ�آ��γɵĻ�������γɹ���____________��
(4)��Ԫ�آߵ�ԭ��������17��Ԫ�������ڱ���λ��Ϊ________________��
(5)д���ɢ٢ܢ�����Ԫ����ɵ�һ�����ӻ�����ĵ���ʽ_______________������������ˮ���ƻ������е�__________(����Ӽ����������ۼ��������Ӽ����ۼ���)��
(6)Ԫ�آٺ�Ԫ�آ��γɵĻ����PԪ�آٺ�Ԫ�آ��γɵĻ������Ϊ18���ӷ��ӣ���д�������ֻ����ﰴ���ʵ���֮��4:1��Ӧ�����ӷ���ʽ________��
���𰸡�Cl- >O2->Mg2+ CH4��NH3��H2O ![]() �������ڵ� ��A��
�������ڵ� ��A�� ![]() ���Ӽ� 4H2O2+H2S=SO42-+2H++4H2O
���Ӽ� 4H2O2+H2S=SO42-+2H++4H2O
��������
����Ԫ�������ڱ���λ�ÿ�ȷ��������H������C������N������O������Na������Mg������P������S������ClԪ�ء�
(1)���Ӻ�����Ӳ���Խ�࣬���Ӱ뾶Խ�����Ӻ�����Ӳ�����ͬʱ���˵����Խ�����Ӱ뾶ԽС��
(2)���������⻯��ķ��Ӽ��������������Ӱ������Ƚϣ�
(3)Mg��O�ȵ�ʧ�����γ����ӣ����Ӽ�ͨ�����Ӽ���ϣ�
(4)Ԫ�آ���PԪ�أ���15��Ԫ�أ�������ԭ��������17��Ԫ����32��Ԫ�أ�����Ԫ�ص�ԭ��������ԭ�ӽṹȷ��Ԫ��λ�ã�
(5)�٢ܢ�����Ԫ�طֱ���H��O��Na������Ԫ���γɵ����ӻ�������NaOH��NaOH����ˮ�������OH-��Na+��
(6)Ԫ�آٺ�Ԫ�آ��γɵĻ�����18���ӷ�����H2O2��Ԫ�آٺ�Ԫ�آ��γɵĻ�����18���ӷ�����H2S�����ᷢ��������ԭ��Ӧ����������ֻ����ﰴ���ʵ���֮��Ϊ4:1��д����Ӧ�����ӷ���ʽ��
(1)�������Ӻ�����Ӳ���Խ�࣬���Ӱ뾶Խ�����Ӻ�����Ӳ�����ͬʱ���˵����Խ�����Ӱ뾶ԽС��Cl-������3�����Ӳ㣬O2-��Mg2+���ⶼ��2�����Ӳ㣬����Cl-�İ뾶���O2-�뾶��֮��Mg2+�����Ӱ뾶��С����Ԫ�آܡ��ޡ�������Ӱ뾶�ɴ�С��˳��ΪCl- >O2->Mg2+��
(2)Ԫ�آڡ��ۡ��ܵļ��⻯��ֱ���CH4��NH3��H2O��CH4ֻ�з��Ӽ���������NH3��H2O�����ڷ��Ӽ��������⣬���������������Ĵ��������˷���֮�����������ʹ���ʵ��۷е����ߣ�����H2O�������NH3�����ǿ��H2O�ķе���ߣ�CH4�ķе���ͣ���������⻯��ķе��ɵ͵��ߵ�˳��ΪCH4��NH3��H2O��
(3)Mgԭ��ʧȥ�����γ�Mg2+��Oԭ�ӻ�õ����γ�O2-��Mg2+��O2-ͨ�����������γ����Ӽ����õ���ʽ��ʾΪ��![]() ��
��
(4)Ԫ�آ���PԪ�أ�����ԭ��������15���ȸ�Ԫ�ص�ԭ��������17��Ԫ����32�����������Ų���2��8��18��4�����Ը�Ԫ��λ�ڵ������ڵ�IVA��
(5)�٢ܢ�����Ԫ�طֱ���H��O��Na������Ԫ���γɵ����ӻ�������NaOH�������ʽ��![]() ��NaOH�����ӻ�����������Ӽ������ۼ�����������ˮ�������OH-��Na+�����Զ��ѵ������Ӽ���
��NaOH�����ӻ�����������Ӽ������ۼ�����������ˮ�������OH-��Na+�����Զ��ѵ������Ӽ���
(6)Ԫ�آٺ�Ԫ�آ��γɵĻ�����18���ӷ�����H2O2�������ʾ��������ԣ�Ԫ�آٺ�Ԫ�آ��γɵĻ�����18���ӷ�����H2S�������ʾ��л�ԭ�ԣ������ʱ�����ʵ���֮��Ϊ4:1����������ԭ��Ӧ�����ݵ����غ㣬�ɵ÷�Ӧ�����ӷ���ʽΪ��4H2O2+H2S=SO42-+2H++4H2O��

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�����Ŀ���Ʊ�������ķ�Ӧԭ�����й��������£�

���� | ��Է������� | ��״ | �۵� | �е� | �ܽ�� |
�ױ� | 92 | ��ɫҺ�� | -95�� | 110�� | ������ˮ |
������ | 122 | ��ɫƬ״����״���� | 122�� | 248�� | ����ˮ |
������� | 158 | ������ˮ |
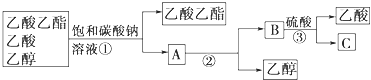
ʵ��������£� �ٽ�������ء�ˮ������������Һ���ҡ�Ⱥ���ױ������õ�Ž��裬���ȣ����¶Ȳ�Ҫ̫�ߣ�����������2h�������и�����ص���ɫ���ڣ���������Ҵ��� �ڽ����Һ���˺���ȴ������Һ�������ữ��������ɫ������ˣ�ϴ�ӣ�����õ�������Ĵֲ�Ʒ�����ⶨ���۵㡣�ش��������⣺
��1�����з�Ӧ�ױ��У���ʵ���в���______������ֹ�������Ҵ���������____________________��
��2�����й��˳��ij�����____________________��
��3�����вⶨ�۵�ʱ�����ֵ�130�� ʱ�����������ۣ��Ʋ�˲�����ijɷ���____________________��
��4���ᴿ������ֲ�Ʒ�ķ�����____________________��
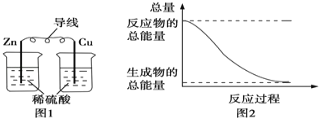
����Ŀ�������ڼ��������¿ɻ�ԭ����ͭ����������ˮ�����⣬����̼�������ij��ѧС��������ͼװ��̽���䷴Ӧ���

[��������]��CO����������Һ��Ӧ��CO��2[Ag(NH3)2]����2OH��===2Ag����2NH4+��CO32����2NH3��
��Cu2OΪ��ɫ������Ag+��Ӧ���ܷ�����Ӧ��Cu2O��2H��===Cu2+��Cu��H2O��
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
��2�������������װ�ô����ҵ�����˳��ΪA��__________________��(����ĸ���)
��3��ʵ���еμ�ϡ����IJ���Ϊ______________________________________________��
��4����֪��������к���CO����װ��C�пɹ۲쵽��������________________��װ��F������Ϊ_________________________________________��
��5������Ӧ������װ��D���Թ��й���ȫ����Ϊ��ɫ��
�����ʵ��֤����ɫ�����к���Cu2O��______________________________________________��
����֤����ɫ�������Ƿ���Cu����ͬѧ�������ʵ�飺��������ɫ�����м�������0.1mol��L1AgNO3��Һ��������Һ�������ݴ��жϺ�ɫ�����к���Cu����ͬѧ��Ϊ�÷�������������֤����ͬѧ�Ľ��ۣ������������¶Ա�ʵ�飬��ɱ������ݡ�
ʵ�鲽��(��Ҫ��д�������������) | Ԥ������ͽ��� |
__________________ | ���۲쵽��Һ����������֤����ɫ�����к���Cu�����۲쵽��Һ����������֤����ɫ�����к���Cu |
����Ŀ�����б�����Ԫ�����ڱ���һ���֣��밴Ҫ�����

��1��Ԫ���������γɵĻ������д��ڵĻ�ѧ������Ϊ__________________
��2��д�����٢ڢ��γɵ�һ��һԪ��ķ���ʽ_________________
��3��д�������γɵĵ��ʵĵ���ʽ_________________
��4���о�Ԫ�����γɵ�������һ����;________________��Ԫ���������ڱ��е�λ�� ____��
��������ŷḻ�ĺ�ˮ��Դ����ˮ��Ԫ���ݡ��������ĺ����ܷḻ��ij��ѧ��ȤС���Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й�����ȡ������Ʒ����ش���������
�ش��������⣺
��5����ˮ�����ķ�����Ҫ��___________________________________(�����о�2��)
��6����������������������Ũ��ˮ�д���Br2�������£�Br2����ɫΪ___________________��
���������ô�����Һ���գ����������Ҫ��Ӧ��Br2+Na2CO3+H2O��NaBr+NaBrO3+ NaHCO3(δ��ƽ)��������1mol Br2ʱ��ת�Ƶĵ�����Ϊ________mol��
��7���Ӻ�ˮ�л��Ԫ���������Ļ������һ�ι���������ͼ��

Ũ��ˮ����Ҫ�ɷ�����:
���� | Na+ | Mg2+ | Cl- | SO42- |
Ũ��/(g��L-1) | 63.7 | 28.8 | 144.6 | 46.4 |
�ù��չ����У���Ʒ1�Ļ�ѧʽΪ________________����Ʒ2ΪMg(OH)2����������Ũ��ˮ�еμ�NaOH��Һ����Mg2+ǡ����ȫ����ʱ��Һ��pHΪ_________��(��֪25��ʱKsp[Mg(OH)2]=1.0��10-13)
��8������MgCl2��6H2O�����Ʊ�MgCl2ʱ��ʵ����ȡ�óɹ��Ĺؼ�������������_________��
����ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪ______________