��Ŀ����
�ۺ����ú�ˮ�����Ʊ�ʳ�Ρ��������þ�����ʣ�����������ͼ��ʾ��
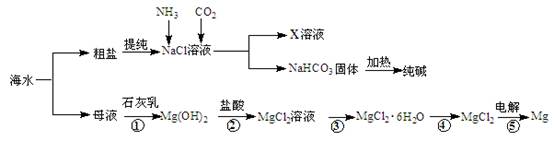
��1����Ӧ�١����У�����������ԭ��Ӧ���� �����ţ���
��2��д����Ӧ�ڵ����ӷ���ʽ ��
��3��X��Һ�е���Ҫ��������Na+�� ��
��4�������к���Na2SO4��MgCl2��CaCl2�ȿ��������ʣ�Ϊ�Ƶô�����NaCl���壬�������£�
���ܽ⣻�����μ��������BaCl2��Һ��NaOH��Һ��Na2CO3��Һ���� ���ܼ���������� �����벹ȫȱ�ٵ�ʵ�鲽�裩
��5�����鴿����Ʒ���Ƿ�NaClӦѡ�õ��Լ��� ��

��1����Ӧ�١����У�����������ԭ��Ӧ���� �����ţ���
��2��д����Ӧ�ڵ����ӷ���ʽ ��
��3��X��Һ�е���Ҫ��������Na+�� ��
��4�������к���Na2SO4��MgCl2��CaCl2�ȿ��������ʣ�Ϊ�Ƶô�����NaCl���壬�������£�
���ܽ⣻�����μ��������BaCl2��Һ��NaOH��Һ��Na2CO3��Һ���� ���ܼ���������� �����벹ȫȱ�ٵ�ʵ�鲽�裩
��5�����鴿����Ʒ���Ƿ�NaClӦѡ�õ��Լ��� ��
��1���� ��2�֣�
��2��Mg (OH)2+2H+ = Mg2+ + H2O ��2�֣�
��3��NH4+��2�֣�
��4������ ��2�֣� �������ᾧ�������𰸺���Ҳ���֣���2�֣�
��5��ϡ���ᡢAgNO3��Һ��2�֣�
��2��Mg (OH)2+2H+ = Mg2+ + H2O ��2�֣�
��3��NH4+��2�֣�
��4������ ��2�֣� �������ᾧ�������𰸺���Ҳ���֣���2�֣�
��5��ϡ���ᡢAgNO3��Һ��2�֣�
�����������1����Ӧ�٢����ڸ��ֽⷴӦ�������ڻ��Ϸ�Ӧ�������ڷֽⷴӦ����Ϊ������ԭ��Ӧ����2������кͷ�Ӧ��Mg (OH)2������ˮд�ɻ�ѧʽ��Mg (OH)2+2H+ = Mg2+ + H2O����3���÷�ӦΪ��ˮ��������̼���Ȼ��Ʒ�Ӧ����̼�����Ƴ������Ȼ�泥�X��Һ�е���Ҫ��������NH4+��Na+����4����������ǰ���ɵij������ܽ������ᣬ��Ӧ���˳�ȥ�������ټ����ᣬȻ��ͨ�������ᾧ�õ��Ȼ��ƾ��壻��5��������Ʒ���Ƿ��������ӣ��������ữ����������

��ϰ��ϵ�д�
 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
�����Ŀ
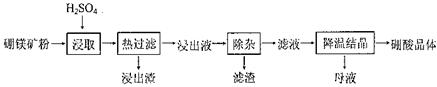
 [B(OH)4]����aq��+H+(aq) K��5.7��10��10��298K��
[B(OH)4]����aq��+H+(aq) K��5.7��10��10��298K��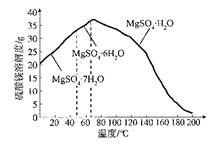







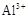 ��pH=5.0ʱ������ȫ��
��pH=5.0ʱ������ȫ�� ��pH=8.8ʱ��ʼ������pH=11.4ʱ������ȫ��
��pH=8.8ʱ��ʼ������pH=11.4ʱ������ȫ��