��Ŀ����
��ѡ�����ʽṹ��.���ǵ��������ḻ�Ľ���֮һ�����γɶ���������ϵ�����ǹ�ҵ�����г��õĴ�����
��1���ϳɰ���ҵʹ�õĴ���������Ϊ����Ķ�ɷִ�����
��NH3��Nԭ�ӵ��ӻ���������� ��
��N��Oͬ���ڶ����ڣ�N�ĵ�һ�����ܱ�O���ԭ���� ��
�۸��ݵȵ�����ԭ����д��һ��NH4���ǵȵ���������Ļ�ѧʽ____________��
��2����ï��[(C5H5)2Fe]��һ�ֽ����л�������ȼ���͵����Ӽ����������ȼ�յ�Ч�ʺ�ȥ�̣�����Ϊ���������ǵ�Ϳ�ϵȡ����Ľṹ������ʾ��������ԭ�ӵĻ�ѧ������ȫ��ͬ��

��Fe�Ļ�̬�����Ų�ʽΪ____________��
�ڶ�ï����Fe2���뻷���ϩ����(C5H5��)֮��Ļ�ѧ�������� _______________��
��1mol�����ϩ�� ���к��ЦҼ�����ĿΪ_________����
���к��ЦҼ�����ĿΪ_________����
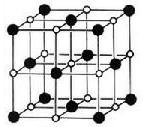
��3����³ʿ���׳��������ṹ��ͼ��ʾ��K+δ����)��ÿ��һ�������������������ĺ���һ��K�����ӣ���³ʿ������Ԫ�صĻ��ϼ���+2��+3���֣�����Fe3����Fe2���ĸ�����Ϊ��_____________��
��1���ϳɰ���ҵʹ�õĴ���������Ϊ����Ķ�ɷִ�����
��NH3��Nԭ�ӵ��ӻ���������� ��
��N��Oͬ���ڶ����ڣ�N�ĵ�һ�����ܱ�O���ԭ���� ��
�۸��ݵȵ�����ԭ����д��һ��NH4���ǵȵ���������Ļ�ѧʽ____________��
��2����ï��[(C5H5)2Fe]��һ�ֽ����л�������ȼ���͵����Ӽ����������ȼ�յ�Ч�ʺ�ȥ�̣�����Ϊ���������ǵ�Ϳ�ϵȡ����Ľṹ������ʾ��������ԭ�ӵĻ�ѧ������ȫ��ͬ��

��Fe�Ļ�̬�����Ų�ʽΪ____________��
�ڶ�ï����Fe2���뻷���ϩ����(C5H5��)֮��Ļ�ѧ�������� _______________��
��1mol�����ϩ��
 ���к��ЦҼ�����ĿΪ_________����
���к��ЦҼ�����ĿΪ_________������3����³ʿ���׳��������ṹ��ͼ��ʾ��K+δ����)��ÿ��һ�������������������ĺ���һ��K�����ӣ���³ʿ������Ԫ�صĻ��ϼ���+2��+3���֣�����Fe3����Fe2���ĸ�����Ϊ��_____________��

�� ��sp3��1�֣�
��Nԭ��ʧȥ��1������������ȶ��İ������2p�ܼ��ϵĵ��ӣ���Ҫ�ṩ�������������Oԭ����ȥ��������2p4���ͣ������2p3���Ͷ����ȶ��Խϲ��1�֣�
��CH4��1�֣�
�� ��[Ar]3d64s2��1s22s22p63s23p63d64s2��1�֣� ����λ����1�֣� ��11NA��1�֣�
�� 1:1��2�֣�
��

��ϰ��ϵ�д�
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
�����Ŀ
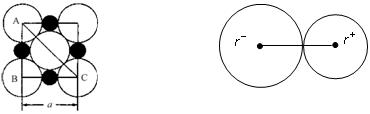
 =_______________��
=_______________��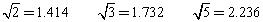 ��
��