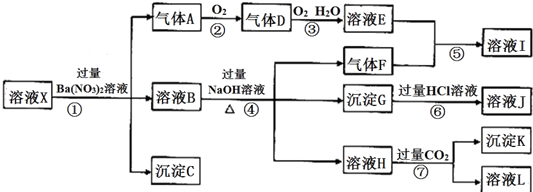
��Ŀ����
6������٤������ԼΪ6.02��1023mol-1�������й�˵����ȷ���ǣ�������| A�� | 1L 0.5mol•L-1 NH4NO3��Һ�к���ԭ����Ŀ6.02��1023 | |
| B�� | ��״���£�22.4L��C3H8����C-H������ĿΪ6.02��1023 | |
| C�� | 32g SO2������O2��ϳ�ַ�Ӧ��ת�Ƶĵ�����ĿΪ6.02��1023 | |
| D�� | �����£�500mL pH=0��������Һ�к��е�H+��ĿΪ6.02��1023 |
���� A��1molNH4NO3�к�2mol��ԭ�ӣ�
B��1molC3H8�к�8molC-H����
C��SO2��O2�ķ�Ӧ��ӦΪ���淴Ӧ�����ܽ��г��ף�
D��pH=0��������Һ��c��H+��=1mol/L��
��� �⣺A��1L 0.5mol•L-1 NH4NO3��Һ��NH4NO3�����ʵ���n=CV=0.5mol/L��1L=0.5mol����1molNH4NO3�к�2mol��ԭ�ӣ���0.5molNH4NO3�к�1mol��ԭ�ӣ���6.02��1023������A��ȷ��
B������£�22.4L��������ʵ���Ϊ1mol����1molC3H8�к�8molC-H�����ʺ��е�C-H���ĸ���Ϊ8��6.02��1023����B����
C��SO2��O2�ķ�Ӧ��ӦΪ���淴Ӧ�����ܽ��г��ף���ת�Ƶĵ�����С��6.02��1023����C����
D��pH=0��������Һ��c��H+��=1mol/L����500mL��������Һ�������ӵ����ʵ���n=CV=0.5L��1mol/L=0.5mol����0.5��6.02��1023������D����
��ѡA��
���� ���⿼���˰���٤���������йؼ��㣬���չ�ʽ�����ú����ʵĽṹ�ǽ���ؼ����ѶȲ���

��ϰ��ϵ�д�
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
�����Ŀ
16������ԭ�ӽṹ��Ԫ�������ɵ�֪ʶ�������ƶ���ȷ���ǣ�������
| A�� | ${\;}_{17}^{35}$Cl2��${\;}_{17}^{37}$Cl2�����������������Ϊͬλ�� | |
| B�� | ${\;}_{34}^{78}$Se��${\;}_{34}^{80}$Se������������ͬ����������ͬ | |
| C�� | ͬ����Ԫ���γɵĺ������������˵���������Ӷ����� | |
| D�� | ͬ��������Ԫ���γɵļ����Ӱ뾶��˵�������������С |
17��ú��ʯ�Dz�ú��ϴúʱ�ķ��������Ҫ�ɷ���Al2O3��SiO2����������������ȵ�Fe2O3��CaO��MgO��Na2O��K2O��P2O5��SO3����ϡ��Ԫ�أ���Ga�ȣ������й���ú��ʯ��˵����ȷ���ǣ�������
| A�� | ������������ʯˮ�ࡢ�ͻ�ש�Ƚ������� | |
| B�� | ú��ʯ�еĽ����������Ϊ���������� | |
| C�� | P2O5��SO3��Ӧˮ��������Խ�ǿ���� H3PO4 | |
| D�� | �����ػ��ã��ܵ��GaCl3ˮ��Һ�Ʊ��ص��� |
14�����н���ʵ����ʵ�ķ���ʽ����ȷ���ǣ�������
| A�� | �����ǵ�������Ӧ����Ũ������������̿�����ڣ�C6H12O6�������ǣ�$\stackrel{Ũ����}{��}$ 6C+6H2O | |
| B�� | ��AgNO3��Һ�м������Na2S��Һ���ټ���NaCl��Һ����ɫ������ɰ�ɫ��Ag2S+2Cl-=2AgCl��+S2- | |
| C�� | ��NH3ͨ����з�̪��ˮ�У���Һ��죺NH3+H2O?NH3•H2O?NH${\;}_{4}^{+}$+OH- | |
| D�� | ������ˮ��ҺpH��7��Al3++3H2O?Al��OH��3�����壩+3H+ |
18��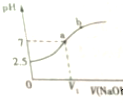 �����£���10mL 0.1mol•L-1 NaHSO3��Һ����εμ�0.1mol•L-1 NaOH��Һ����Ӧ��������ҺpH�仯��ͼ��ʾ������˵������ȷ���ǣ�������
�����£���10mL 0.1mol•L-1 NaHSO3��Һ����εμ�0.1mol•L-1 NaOH��Һ����Ӧ��������ҺpH�仯��ͼ��ʾ������˵������ȷ���ǣ�������
 �����£���10mL 0.1mol•L-1 NaHSO3��Һ����εμ�0.1mol•L-1 NaOH��Һ����Ӧ��������ҺpH�仯��ͼ��ʾ������˵������ȷ���ǣ�������
�����£���10mL 0.1mol•L-1 NaHSO3��Һ����εμ�0.1mol•L-1 NaOH��Һ����Ӧ��������ҺpH�仯��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | NaHSO3��Һ������ | B�� | V1��10mL | ||
| C�� | ��a�㣬c��Na+��=c��SO32- ��+c��HSO3-�� | D�� | HSO3-�ĵ��볣��Ka=6.25��10-7 |
16���������ƣ�NaNO2����һ�ֳ�����ʳƷ���Ӽ���ʹ��ʱ�����ϸ������������ij��ȤС���������ʵ��̽������������֪����
��2NO+Na2O2=2NaNO2��2NO2+Na2O2=2NaNO3
������KMnO4��Һ�ɽ�NO2-����ΪNO3-��MnO4-��ԭ��Mn2+��
��Ʒ�Ʊ�����飺����ͼ1װ���Ʊ�NaNO2��
��1��д��װ��A��ƿ�з�����Ӧ�Ļ�ѧ����ʽ���������ת�Ƶķ������Ŀ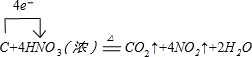 ��
��
��2��Bװ�õ������ǽ�NO2ת��ΪNO��ͬʱCu��ϡ���ᷴӦ����NO������ȡNO����
��3����ͬѧ��Ϊװ��C�в��ﲻ�����������ƣ�����̼���ƺ��������ƣ�Ϊ�Ʊ�����NaNO2Ӧ��B��Cװ�ü�����һ��װ�ã������ҿ��ڻ������ӵ�װ��ͼ2��������ʢ�ŵ��Լ���
��4�������ʵ�����װ��C��NaNO2�Ĵ��ڣ�д������������ͽ��ۣ�ȡ����װ��C�в��������Թ��У�������������ˮ�ܽ⣬������ϡ�����ữ���μ���1-2�Σ�����������KMnO4��Һ������Һ��ɫ��ȥ��˵��C�в��ﺬ��NaNO2��
�����IJⶨ
��ȡװ��C�з�Ӧ��Ĺ���4.000g����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000mol/L����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
��5����һ��ʵ�����ݳ����쳣����������쳣��ԭ�������AC��˫��ѡ��
A����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
B����ƿϴ����δ����
C���ζ��������Ӷ���
D���ζ����˸��Ӷ���
��6�����ݱ������ݣ��������ù������������Ƶ���������86.25%��0.8625��
���������4λ��Ч���֣�
��2NO+Na2O2=2NaNO2��2NO2+Na2O2=2NaNO3
������KMnO4��Һ�ɽ�NO2-����ΪNO3-��MnO4-��ԭ��Mn2+��
��Ʒ�Ʊ�����飺����ͼ1װ���Ʊ�NaNO2��

��1��д��װ��A��ƿ�з�����Ӧ�Ļ�ѧ����ʽ���������ת�Ƶķ������Ŀ
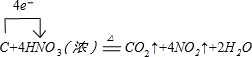 ��
����2��Bװ�õ������ǽ�NO2ת��ΪNO��ͬʱCu��ϡ���ᷴӦ����NO������ȡNO����
��3����ͬѧ��Ϊװ��C�в��ﲻ�����������ƣ�����̼���ƺ��������ƣ�Ϊ�Ʊ�����NaNO2Ӧ��B��Cװ�ü�����һ��װ�ã������ҿ��ڻ������ӵ�װ��ͼ2��������ʢ�ŵ��Լ���
��4�������ʵ�����װ��C��NaNO2�Ĵ��ڣ�д������������ͽ��ۣ�ȡ����װ��C�в��������Թ��У�������������ˮ�ܽ⣬������ϡ�����ữ���μ���1-2�Σ�����������KMnO4��Һ������Һ��ɫ��ȥ��˵��C�в��ﺬ��NaNO2��
�����IJⶨ
��ȡװ��C�з�Ӧ��Ĺ���4.000g����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000mol/L����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
| ����� | 1 | 2 | 3 | 4 |
| KMnO4��Һ���/mL | 20.60 | 20.02 | 20.00 | 19.98 |
A����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
B����ƿϴ����δ����
C���ζ��������Ӷ���
D���ζ����˸��Ӷ���
��6�����ݱ������ݣ��������ù������������Ƶ���������86.25%��0.8625��
���������4λ��Ч���֣�