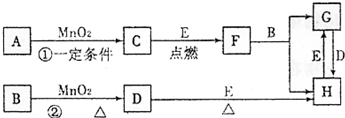
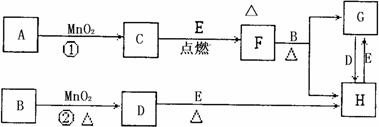
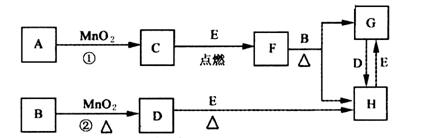
��Ŀ����
��ͼ�漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ�����C��D��EΪ���ʣ�����Ϊ���������Һ�����Ǵ�������ת����ϵ����Ӧ�����ɵ�ˮ����Ҫ���������ȥ��(1)д����ѧʽ��B______________��E_____________��
(2)ָ��MnO2����ط�Ӧ�е����ã���Ӧ������_________������Ӧ������__________����
(3)���F��B������Ӧ��ѧ����ʽ_______________________��
(4)����Ӧ�����ڼ��������½��У�A��________________������Ӧ�����ڳ��������½��У�A��__________________�����������������µõ�������C���ʣ���Ӧ��ת�Ƶĵ�����֮��Ϊ___________________��
(1)HCl��Fe��(2)���������� (3)Fe3O4+8HCl====FeCl2+2FeCl3+4H2O��
(4)KClO3��H2O2��2��1

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| |||||||||||||||||||