��Ŀ����
�����й��к��ȵ�˵����ȷ����(����)
| A����ʾ�к��ȵ��Ȼ�ѧ����ʽ��H��(l)��OH��(l)=H2O(l)����H����57.3 kJ/mol |
| B��ȷ�����к��ȵ�ʵ������У�������ⶨ�¶�4�� |
| C���к��ȵ�ʵ������У����β����������������ͭ���棬����������к�����ֵƫС |
| D����֪2NaOH(aq)��H2SO4(aq)=Na2SO4(aq)��2H2O(l)����H����114.6 kJ/mol����÷�Ӧ���к���Ϊ114.6 kJ/mol |
C
�������������A��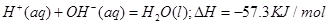 ������B����Ҫ�ⶨ�¶����Σ���ƽ��ֵ������C��ͭ˿�����Ժã���ʹ����ɢ����ȥ��ʹ����ֵƫС����ȷ��D����Ӧ���к���������1mol
������B����Ҫ�ⶨ�¶����Σ���ƽ��ֵ������C��ͭ˿�����Ժã���ʹ����ɢ����ȥ��ʹ����ֵƫС����ȷ��D����Ӧ���к���������1mol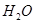 �ų������������Ը÷�Ӧ���к���Ϊ-57.3KJ/mol,����
�ų������������Ը÷�Ӧ���к���Ϊ-57.3KJ/mol,����
���㣺�к��ȡ�

 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�����ͼ��ֱ��ʾ�йط�Ӧ�ķ�Ӧ�����������仯�Ĺ�ϵ��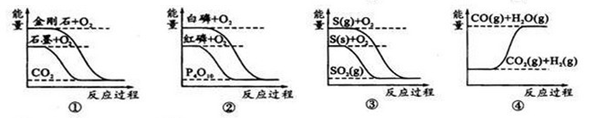
�ݴ��ж�����˵����ȷ����
| A��ʯīת��Ϊ���ʯ�����ȷ�Ӧ |
| B�����ױȺ����ȶ� |
| C��CO(g)+H2O(g)=CO2(g)+H2(g)��H��0 |
| D��S(g)+O2(g)=SO2(g) ��H1�� S(s)+O2(g)=SO2(g) ��H2����H1����H2 |
��֪��2H2(g)��O2(g)��2H2O(l) ��H����571.6 kJ��mol��1 ��
2CH3OH(l)��3O2(g)��2CO2(g)��4H2O(l) ��H����1452 kJ��mol��1 ��
H��(aq)��OH��(aq)��H2O(l) ��H����57.3 kJ��mol��1 ����˵����ȷ���ǣ� ��
| A��H2(g)��ȼ����Ϊ571.6 kJ��mol��1 |
| B��ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų��������� |
C�� H2SO4(aq)�� H2SO4(aq)��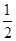 Ba(OH)2(aq)�� Ba(OH)2(aq)�� 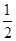 BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 |
| D��3H2(g)��CO2(g)�� CH3OH(l)��H2O(l)����H����135.9 kJ��mol��1 |
�к��Ȳⶨʵ���У���50mL0.50mol/L�����50mL0.55mol/LNaOH����ʵ�飬����˵��������ǣ� ��
| A������60mL 0.50mol/L�����50mL 0.55 mol/L NaOH��Һ���з�Ӧ��������к�����ֵ��ԭ����ͬ |
| B����50mL0.50mol/L�����50mL0.55mol/LNaOH����ʵ�����50mL0.50mol/L����50mL0.50mol/LNaOH��õ���ֵȷ |
| C�������ʱ����Ͳ��NaOH��ҺӦ��������С�ձ��У������ò��������� |
| D��װ���еĴ�С�ձ�֮����������ĭ���ϵ������DZ��¸��ȡ�����������ʧ |
ij��Ӧ�ķ�Ӧ�����������仯��ͼ��ʾ(ͼ��E��ʾ���)������������ȷ����( )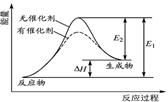
| A���淴Ӧ�Ļ�ܴ�������Ӧ�Ļ�� |
| B���÷�ӦΪ���ȷ�Ӧ |
| C�������ܸı䷴Ӧ���ʱ� |
| D�������ܽ��ͷ�Ӧ�Ļ�� |
SF6��һ�������ľ�Ե���壬���ӽṹ��ֻ����S��F������֪��1 mol S(s)ת��Ϊ��̬��ԭ����������280 kJ������1 mol F��F��S��F�������յ������ֱ�Ϊ160 kJ��330 kJ����S(s)��3F2(g)=SF6(g)�ķ�Ӧ�Ȧ�HΪ(����)
| A����1 780 kJ/mol | B����1 220 kJ/mol | C����450 kJ/mol | D����430 kJ/mol |
�����й��Ȼ�ѧ����ʽ��������ȷ���ǣ� ��
| A����֪2H2��g����O2��g��= 2H2O��g������H=��483��6 kJ/mol����������ȼ����Ϊ241��8 kJ/mol |
| B����֪C��ʯī��s��= C�����ʯ��s������H��0������ʯ��ʯī�ȶ� |
| C����20��0g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28��7 kJ����������÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽΪ��NaOH��aq����HCl��aq��=NaCl��aq����H2O��aq������H=��57.4 kJ/mol |
| D����֪2C��s����2O2��g��=2CO2��g������H1��2C��s����O2��g��=2CO��g�� ����H2�����H1<��H2 |
����ƽ�����ʢ��Һ̬��(N2H4)��Һ̬˫��ˮ�������ǻ��ʱ����������������ˮ���������ų��������ȡ���֪6.4 gҺ̬����������Һ̬˫��ˮ��Ӧ���ɵ�����ˮ����ʱ���ų�128.326 kJ�������������Ȼ�ѧ����ʽ��ȷ����(����)
| A��N2H4(l)��2H2O2(l)=N2(g)��4H2O(g) ��H��128.326 kJ��mol��1 |
| B��N2H4(l)��2H2O2(l)=N2(g)��4H2O(g) ��H����128.326 kJ��mol��1 |
| C��N2H4(l)��2H2O2(l)=N2(g)��4H2O(g) ��H����641.63 kJ��mol��1 |
| D��N2H4(l)��2H2O2(l)=N2(g)��4H2O(g) ��H����256.625 kJ��mol��1 |
����˵����ȷ����
| A�����ȷ�Ӧ�ڳ�����һ�����ܷ��� |
| B�����ȷ�Ӧ�ķ�Ӧ�������Ǵ������ȷ�Ӧ�ķ�Ӧ���� |
| C����֪C(ʯī��s)=C(���ʯ��s)����H1>0��˵��ʯī�Ƚ��ʯ�ȶ� |
D��ͬ��ͬѹ�£�H2(g)��Cl2(g)=2HCl(g)�ڹ��պ͵�ȼ������ ��ͬ ��ͬ |