��Ŀ����
MnO2��һ����Ҫ�Ĵ�����ij�о���ѧϰС������˽���MnO2�����н϶��MnO��MnCO3����Ʒת��Ϊ��MnO2ʵ�飬���������£�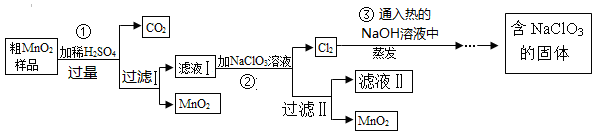
��1��д��1���ö��������������Ļ�ѧ��Ӧ����ʽ ��
��2���ڢڲ���Ӧ�����ӷ�Ӧ����ʽΪ ��
��3��������ˢ����õ�MnO2�Ƿ�ϴ�Ӹɾ��ķ����� ��
��4���ڢ۲���Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��5�����������п�����ѭ��ʹ�õ������� ���ѧʽ�������˲��������������ж�Ҫ�õ��IJ��������� ��
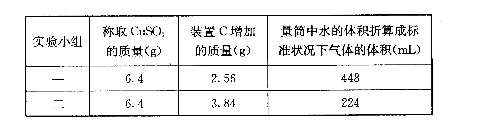
��6������MnO2��Ʒ������Ϊ25.38g���ڢٲ���Ӧ�����˵õ�17.4g MnO2�����ռ���0.448LCO2����״���£�������Ʒ��������MnO����Ϊ g��
��1��2KClO3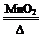 2KCl��3O2����2H2O2
2KCl��3O2����2H2O2 2H2O��O2��
2H2O��O2��
��2��5Mn2++2ClO3-+4H2O��Cl2��+5MnO2+8H+
��3��ȡ���һ��ϴ��Һ���Թ��У��μ��Ȼ�����Һ��������������֣�˵����ϴ�Ӹɾ�
��4��3Cl2+ 6NaOH NaClO3 + NaCl + 3H2O ��5��NaClO3������������1�֣� ��6��5. 68g
NaClO3 + NaCl + 3H2O ��5��NaClO3������������1�֣� ��6��5. 68g
���������������1��ʵ��������ȡ����ʱ���ö�����������������Ӧ�Ļ�ѧ����ʽΪ2KClO3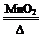 2KCl��3O2����2H2O2
2KCl��3O2����2H2O2 2H2O��O2����
2H2O��O2����
��2������ؾ���ǿ�����ԣ��ܰ���Һ�е�Mn2+�������ɶ������̣�������صĻ�ԭ��������������Ӧ�����ӷ���ʽΪ5Mn2++2ClO3-+4H2O��Cl2��+5MnO2+8H+��
��3����Һ�к���SO42��������������������SO42������˿���ͨ������SO42����������������Ƿ�ϴ�Ӹɾ���������ȷ��ʵ�������ȡ���һ��ϴ��Һ���Թ��У��μ��Ȼ�����Һ��������������֣�˵����ϴ�Ӹɾ���
��4���������ȵ�����������Һ��Ӧ���������ơ��Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ3Cl2+ 6NaOH NaClO3 + NaCl + 3H2O��
NaClO3 + NaCl + 3H2O��
��5���ɹ�������ͼ���Կ�����ѭ�����õ���NaClO3����ǰ����Ϊ��Ӧ����˺��������������������ʿ���ѭ��ʹ�á����˲��������������ж�Ҫ�õ��IJ��������Dz�������
��6����״����CO2�����ʵ�����0.448L��22.4L/mol��0.02mol�������̼ԭ���غ��֪̼���̵����ʵ�����0.02mol��������0.02mol��115g/mol��2.3g����ԭ�������MnO��������25.38g��17.4g��2.3g��5.68g��
���㣺���������Ʊ������ʵķ������ᴿ�����ʺ����ļ����Լ�ʵ�鷽����Ƶ�

 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
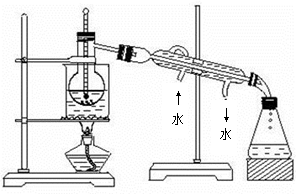
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д���Ԫ�ض��˵Ľ����dz���Ҫ�������ǴӺ������з�������ʵ⣨I2��������ͼ��
��1�����̢ٵ�ʵ�����������____________��____________��
��2�����������У�����������ԭ��Ӧ����_______�����ӷ���ʽΪ ��
��3�����̢۵�ʵ�����������___________��___________���ڴ˹����У��ɹ�ѡ�����
���ܼ���_______������ĸ���ţ���
| A���ƾ� | B��CCl4 | C���� | D������ |
�� ��
�� ��
�� ��
�� �����м����������������������۷֡���
ʵ������ȡ��ϩ�ķ�Ӧԭ��Ϊ��CH3CH2OH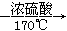 CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������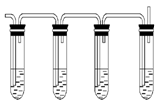
�Իش��������⣺
��1��ͼ�Т٢ڢۢ�װ��ʢ�ŵ��Լ��ֱ��ǣ���ѡ����ĸ������_________����_________��
| A��Ʒ����Һ | B��NaOH��Һ | C��Ũ���� | D�����Ը��������Һ |
��3��ȷ֤��ϩ���ڵ�������_______________________________________________________________��
������������(FeC2O4��2H2O)�ʵ���ɫ��ij������Ϊ̽������ҵ������Ļ�ѧ���ʣ� ������һϵ��ʵ��̽����
(1)��ʢ�в�������������Թ��е��뼸�������ữ��KMnO4��Һ����������Һ��ɫ��Ϊ�ػ�ɫ������������̼�������ɡ���˵����������������� (������ԡ�������ԭ�ԡ����ԡ�)������Ӧ������1 mol FeC2O4��2H2O����μӷ�Ӧ��KMnO4Ϊ mol��
(2)���ϱ��������ܱ������м��ȵ�һ���¶�ʱ�����������������ȫ�ֽ⣬���ɼ��������������Ϊ��ɫ���塣��������ݿα��������ܵ���������������ʣ��Ժ�ɫ��������������¼��裬������ɼ�����ͼ�������
����һ��ȫ����FeO
�������
��������
(3)Ϊ��֤��������һ�Ƿ��������������������о���
�������о�����������±������ݡ�
| ʵ�鲽��(��Ҫ��д�������������) | Ԥ��ʵ������ͽ��� |
| ȡ������ɫ���壬 | |
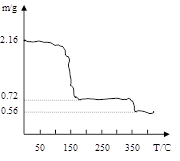
�������о����������������в��ĵ���FeC2O4��2H2O���ȷֽ�ʱ�������������¶ȱ仯����������ͼ��ʾ��д�����ȵ�400��ʱ��FeC2O4��2H2O�������ȷֽ�Ļ�ѧ����ʽΪ�� ��
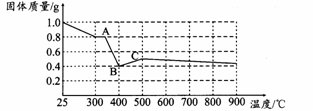
����ͼ������1.0 g�������������������г��ڳ�ּ��ȣ����ղ�����ɫ�������������0.4 g��ijͬѧ�ɴ˵ó����ۣ�����һ�����������Ƿ�ͬ���ͬѧ�Ľ��ۣ����������ɣ�
��ҵ����ƽ�VOSO4�е�K2SO4��SiO2���ʳ�ȥ�����յõ�V2O5���������£�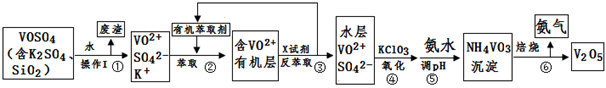
��ش��������⣺
��1����������÷����ijɷ��� ��д��ѧʽ��������I������ ��
��2������ڡ��۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO2+��HA��ʾ�л���ȡ������
R2(SO4)n (ˮ��)+ 2nHA���л��㣩 2RAn���л��㣩 + nH2SO4 (ˮ��)
2RAn���л��㣩 + nH2SO4 (ˮ��)
������ȡʱ��������������ԭ���� ��
����X�Լ�Ϊ ��
��3���ݵ����ӷ���ʽΪ ��
��4��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
����ϱ�����ʵ�������У����м��백ˮ��������Һ�����pHΪ ������������Ϊ93.1%ʱ������Fe(OH)3����������Һ��c(Fe3+)< ������֪��25��ʱ��Ksp[Fe(OH)3]=2.6��10��39��
��5���ù��������У�����ѭ�����õ������� �� ��