��Ŀ����
�����״��� ����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״��ĺϳ�������ͼ1��ʾ��
����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״��ĺϳ�������ͼ1��ʾ��
��֪��i)�����Լ�����ˮ�⣬
ii)������ʵ������������£�
| ���� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� |
| �����״� | 164.2 | 380 | ������ˮ�������Ҵ������ѵ��л��ܼ� |
| ���� | ��116.3 | 34.6 | ����ˮ�������Ҵ��������л��ܼ� |
| �屽 | ��30.7 | 156.2 | ������ˮ�������Ҵ������ѵȶ����л��ܼ� |
| ���������� | ��34.6 | 212.6 | ������ˮ |
| Mg(OH)Br | ������Ϊ���� | ������ˮ�������ڴ����ѵ��л��ܼ� | |
iii)�����״�����Է���������260�����������л���һ�㶼�й̶��۵㡣
��ش��������⣺
��1��ʵ���Һϳ������״���װ��ͼ2��д����������A�����ƣ� ��װ����ˮCaCl2������A�������ǣ� ��

��2����ȡ�����Լ�ʱҪ�����У����Բ���ˮԡ���ȣ��ŵ��� ���л���ʱ��������ˮ���ķ����ǣ� ���X��Y����Y��X������
��3���Ƶõ������״��ֲ�Ʒ�У��������ѡ��屽���������������л���ͼ�ʽ�廯þ�����ʣ�������������ᴿ����������д�հף�

���У�������Ϊ�� ��ϴ��Һ���ѡ�ã� ��
A��ˮ B������ C���Ҵ� D����
�����Ʒ�Ѿ�ϴ�Ӹɾ��IJ���Ϊ�� ��
��4�����Ȳⶨ����ȡ2.60g��Ʒ�����������Һ���������������ƣ��������Ʋ��ᷴӦ������ַ�Ӧ��������������Ϊ100.80m L(��״��������Ʒ�������״���������Ϊ ��������λ��Ч���֣���֪�����״��ķ���ʽΪC19H16O����Է�������Ϊ260����
��18�֣���1������ܣ�2�֣� ��ֹ������ˮ��������װ�ã�2�֣�
��2�����Ⱦ��ȣ��¶����ڿ��ƣ�2�֣� X��Y��2�֣�
��3���������2�֣� A��2�֣� ȡ���һ��ϴ��Һ���Թ��У��μ�AgNO3��Һ�����ް�ɫ�������ɣ����Ѿ�ϴ�Ӹɾ���3�֣�
��4��90%��3�֣�
���������������1����ͼ2�ɵã�A�Ǹ���ܣ���Ϊ�����Լ�����ˮ�⣬��ˮ�Ȼ��������Ը��������Ҫ�Ƿ�ֹ������ˮ��������װ�ã� ��ֹ�����Լ�ˮ�⣬���������״��IJ������ͣ���2������ˮ�ķе�Ϊ100�棬ˮԡ���Կ��Ƽ��ȵ��¶Ȳ�����100�棬�ҿ���ʹ��Ӧ�������ȣ�����������ȴˮ��ȡ����ԭ����ˮ�������ǵͽ��߳�������X��Y�ķ�����������3������֪��Ϣii)���й����ʵ��������ʣ������״������ѡ��屽�������������ǻ���Һ�����������ɳɷֵķе����ϴ���˴ֲ�Ʒ����ķ������������������������������Ŀ���dz�ȥ���ѡ��屽�����������������ʣ����ڼ�ʽ�廯þ������ˮ�������ڴ����ѵ��л��ܼ���������ڵ�Ŀ���dz�ȥ��ʽ�廯þ�����������״�������ˮ�������Ҵ������ѵ��л��ܼ������ϴ��Һ���ѡ��ˮ������ѡ�����ѡ��Ҵ��������л���Һ����ֹ���������ѡ��Ҵ��������µ����ʣ������ˮ��������ˮ�Ȼ��Ƶȸ������ȥ���ٴ����ɵõ������������״������ںϳ�����ͼ�м����Ȼ�隣�����Һ����������Ҫ�ɷ��Ǽ�ʽ�廯þ�����������������������ӵ����ʣ������������������������ӻ�笠����ӣ����������ӵ����ʿ������ʵ�鷽����������Ƿ�ϴ�Ӹɾ�����ȡ���һ��ϴ��Һ���Թ��У��μ�AgNO3��Һ�����ް�ɫ�������ɣ����Ѿ�ϴ�Ӹɾ�����4���������������=100.80mL=0.1008L����״��������Ħ�����Ϊ22.4L/mol��n=V/Vm�������������ʵ���=0.1008L��22.4L/mol�����������״�ֻ����1���ǻ�������2mol�ǻ���2molNa�����û���Ӧ������1molH2����1mol�����״�������Na��Ӧ���ų�0.5mol H2���������״������ʵ�����������2�����������״������ʵ���Ϊ0.1008L��22.4L/mol��2�����������״��ķ���ʽΪC19H16O����Է�������Ϊ260��m=n?M�����Ʒ�������״�������Ϊ0.1008L��22.4L/mol��2��260g/mol=2.34g�����ڲ�Ʒ������Ϊ2.60g�����Ʒ�������״�����������Ϊ2.34g��2.60g��100%=90%��
���㣺�����ۺ�ʵ�鼰��ѧ���㣬�漰�������������ơ�����������á�ˮԡ���ȵ��ŵ㡢����������ȴˮ�������������ᴿ�ķ�����ϴ��Һ��ѡ����ϴ�Ӹɾ��ķ��������ʵ����ڻ�ѧ����ʽ�е�Ӧ�á��������������Ħ����������ʵ�����Ħ�������������״��������ʹ��ȼ��㡢��Ч���ֵĴ����ȡ�

��֪ij��ȼ�Ϻ���̼���⡢������Ԫ�ء�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������������ȼ�գ���ʹ����������ȫ��ͨ����ͼ��ʾ��װ�ã��õ����±����е�ʵ����(���������������ȫ������)��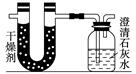
| | ʵ��ǰ | ʵ��� |
| (�������U�ι�)������ | 101.1 g | 102.9 g |
| (ʯ��ˮ�����ƿ)������ | 312.0 g | 314.2 g |
����ʵ��������
(1)ʵ����Ϻ���������ˮ������Ϊ________ g��
������ƿ������һ�����Σ�������Ϊ________ g��
(2)���ɵ�ˮ����Ԫ�ص�����Ϊ________ g��
(3)���ɵĶ�����̼��̼Ԫ�ص�����Ϊ________ g��
(4)��ȼ����̼Ԫ������Ԫ�ص�������Ϊ________��
(5)��֪���ִ���ÿ�������к���һ����ԭ�ӣ���ô��ķ���ʽΪ__________���ṹ��ʽΪ_______________________________��
���й�����ϩ�;���ϩ����������ȷ����
| A����ϩ�����������壬Ϊ���������ϩ�������ǹ��壬Ϊ����� |
| B����ϩ�Ļ�ѧ���ʱȾ���ϩ���� |
| C��ȡ����������ϩ�;���ϩ��ȫȼ�պ����ɵ�CO2��H2O�������ֱ���� |
| D��ȡ�����ʵ�������ϩ�;���ϩ��ȫȼ�պ����ɵ�CO2��H2O�����ʵ����ֱ���� |
���й���ǰ�����������仯���������ڻ�ѧ�仯����
| A��̼���ƾ���ķ绯������ͭ����ˮ |
| B��ú�ĸ���ʯ�͵��ѻ� |
| C��������������ڵ��Ȼ�þ���� |
| D��ú���������ý��ݹ����������Һ�Ĺ���������ˮ�� |
���и���Ӧ�����ڼӳɷ�Ӧ���ǣ� ��
A��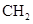  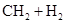 �� ��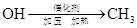 �� ��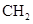 �� ��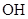 |
B��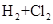  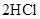 |
C��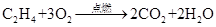 |
D��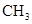 �� ��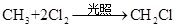 �� ��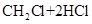 |
�Ӹ̽��������ƶ�ϩ  �����е��й������Ʋ⣬����ȷ����
�����е��й������Ʋ⣬����ȷ����
| A������ʽΪC10H16 |
| B��������ΪҺ̬��������ˮ |
| C��������ʹ���Ը��������Һ��ɫ |
D���������������Ȼ�̼��Һ��Ӧ�����Ϊ |



