��Ŀ����
�����ʵ���Ũ�Ⱦ�Ϊ0.1mol��L��1��HA��BOH��Һ�������ϣ�����˵���������
| A����HAΪ���ᣬBOHΪ�������C(H��)+C(B��)===C(OH��)+ C(A��) |
| B����HAΪǿ�ᣬBOHΪ�������C(A��)��C(B��) ��C(H��) ��C(OH��) |
| C����HAΪ���ᣬBOHΪǿ�����C(B��) ��C(A��) ��C(OH��) ��C(H��) |
| D����HAΪǿ�ᣬBOHΪǿ�����C(H��)= C(A��)= C(B��)= C(OH��)=0.1mol��L��1 |
D
���������D��������ϣ���Һ����������˶�����Ũ����С�˶�������C(H��)= C(A��)= C(B��)= C(OH��)=0.05mol��L��1���ʴ�����ѡD��
���������⿼������Ũ�ȵĴ�С�Ƚϣ���Ŀ�Ѷ��еȣ�������Ĺؼ��Ǹ�����Һ��pH����Һ���������������������жϵ���ʵ�ǿ�����������ˮ���ԭ���ɽ����⡣

��ϰ��ϵ�д�
�����Ŀ

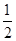 O2(g) = H2O(1)��H�� �D285.8kJ��mol
O2(g) = H2O(1)��H�� �D285.8kJ��mol =10-12������������Һ�������ϣ�������Һ���������ʵ���Ũ�ȹ�ϵ����ȷ����( )
=10-12������������Һ�������ϣ�������Һ���������ʵ���Ũ�ȹ�ϵ����ȷ����( )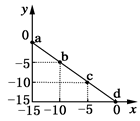
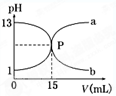
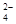 )��c(H��)ԼΪ(����)��
)��c(H��)ԼΪ(����)��