��Ŀ����
CO2�������г����Ļ�����֮һ���������繤ҵ���õķ�չ���˿ڵľ�����ȫ����Դ���ż�������������Խ��Խ���ص����⣬�����CO2�������о����ۺ������������ӡ�
���ڴ��������£����ɼ״���CO2ֱ�Ӻϳ�̼�������(DMC)��CO2 + 2CH3OH �� CO(OCH3)2 + H2O�����״�ת����ͨ�����ᳬ��1%��������Լ�÷�Ӧ����ҵ������Ҫԭ��ij�о�С���������������������£�ͨ���о��¶ȡ���Ӧʱ�䡢���������ֱ��ת����(TON)��Ӱ�������۴����Ĵ�Ч�������㹫ʽΪ��TON��ת���ļ״������ʵ���/���������ʵ�����
��1�����ݷ�Ӧ�¶ȶ�TON��Ӱ��ͼ����ͬʱ���ڲⶨ�����жϸ÷�Ӧ���ʱ��H________0�����������������������������____________________________________��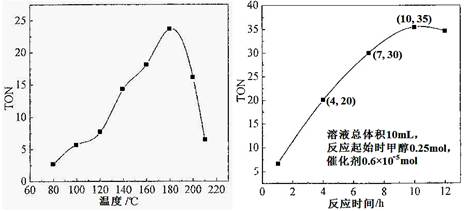
��2�����ݷ�Ӧʱ���TON��Ӱ��ͼ����ͼ������֪��Һ�����10mL����Ӧ��ʼʱ�״�0.25mol������0.6��10��5 mol��������¶��£�4��7 h��DMC��ƽ����Ӧ���ʣ�________��
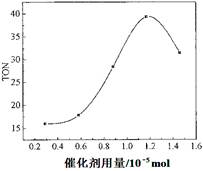
��3�����ݸ��о�С���ʵ�鼰����������TON��Ӱ��ͼ��������ͼ�����ж�����˵����ȷ���� ��
A���ɼ״���CO2ֱ�Ӻϳ�DMC���������ü״���Ӱ�컷������������CO2 ת��Ϊ��Դ������Դѭ�����úͻ����������涼������Ҫ����
B. �ڷ�Ӧ��ϵ�����Ӻ��ʵ���ˮ��������߸÷�Ӧ��TON
C. ��������������1.2��10��5 molʱ�����Ŵ������������ӣ��״���ƽ��ת�����������
D. ��������������1.2��10��5 molʱ�����Ŵ�������������DMC�IJ��ʷ��������½�
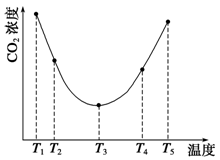
��.��������ڵ�CO2����˳���ų������������ж���������Һ���Ե������������������ҺpH��Ӱ�죬����ѪҺ����Ҫͨ��̼�����λ�����ϵ(H2CO3/HCO3-)ά��pH�ȶ�����֪��������ѪҺ����������ʱ��H2CO3��һ�����볣��Ka1=10-6.1��c(HCO3-):c(H2CO3)��20:1��lg2=0.3��
(4)��������ݿ������������ѪҺ��pH________(����һλС��)��
(5)��������ѪҺ��H2CO3��HCO3-��OH-��H+������Ũ���ɴ�С��ϵΪ�� ��
(6)���������ᡢ�����ѪҺ��ʱ��ѪҺpH�仯������ԭ�������������������������� ��
��1������ ������ͬ�ķ�Ӧʱ�䣬�¶Ƚϵ�ʱ����Ӧδ�ﵽƽ�⣻�¶Ƚϸ�ʱ����Ӧ�Ѵﵽƽ�⣬�����¶����ߣ�TON��С����ƽ�������ƶ���˵���÷�Ӧ���� ��2��1��10-3 mol��L-1��h-1
��3��AB (4) 7.4 (5)c(HCO3-)��c(H2CO3)��c(OH-)��c(H+)
(6)�����������ѪҺ��ʱ��HCO3-����H+������Ӧ��ά��ѪҺ��pH�ȶ��������������ѪҺ��ʱ��H2CO3����OH_������Ӧ��ά��ѪҺ��pH�ȶ�
���������������1������ͼ���֪��������ͬ�ķ�Ӧʱ�䣬�¶Ƚϵ�ʱ����Ӧδ�ﵽƽ�⣻�¶Ƚϸ�ʱ����Ӧ�Ѵﵽƽ�⣬�����¶����ߣ�TON��С����ƽ�������ƶ���˵���÷�Ӧ���ȡ�
��2������ͼ���֪��4��7 h��DMC�仯��Ϊ30��20��10�������TON��ת���ļ״������ʵ���/���������ʵ�����֪��ת���ļ״����ʵ�����0.6��10��5 mol��10��6��10��5 mol����Ũ����6��10��5 mol��0.01L��6��10��3 mol/L������DMC��Ũ����3��10��3 mol/L������4��7 h��DMC��ƽ����Ӧ���ʣ�3��10��3 mol/L��3h��1��10-3 mol��L-1��h-1��
��3��A���ɼ״���CO2ֱ�Ӻϳ�DMC���������ü״���Ӱ�컷������������CO2 ת��Ϊ��Դ������Դѭ�����úͻ����������涼������Ҫ���壬A��ȷ��B���ڷ�Ӧ��ϵ�����Ӻ��ʵ���ˮ�������Խ���������ˮ������Ũ�ȣ���ʹƽ��������Ӧ������У��״���ת����������˽���߸÷�Ӧ��TON��B��ȷ��C������TON��ת���ļ״������ʵ���/���������ʵ�����֪��TON��״�ת�������Լ������������й�ϵ�����Ե�������������1.2��10��5 molʱ�����Ŵ������������ӣ��״���ƽ��ת���ʲ�һ������ߣ�C����ȷ��D���������ܸı�ƽ��״̬�����DMC�IJ����������ϵ��D����ȷ����ѡAB��
��4��Ka��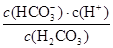 ��Ka=10-6.1mol?L-1������ѪҺ��c��HCO3-����c��H2CO3����20��1����c��H+��=10-7.4mol?L-1����pH��7.4��
��Ka=10-6.1mol?L-1������ѪҺ��c��HCO3-����c��H2CO3����20��1����c��H+��=10-7.4mol?L-1����pH��7.4��
��5�����ݣ�4���з�����֪��������ѪҺ�Լ��ԣ�c(OH-)��c(H+)����֪Ka��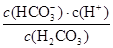 ��10-6.1��ѪҺ��ƷpHΪ7.4����c��H+��=10-7.4mol/L������
��10-6.1��ѪҺ��ƷpHΪ7.4����c��H+��=10-7.4mol/L������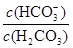 ��10?6.1��10?7.4��101.3�����ѪҺ��H2CO3��HCO3-��OH-��H+������Ũ���ɴ�С��ϵΪc(HCO3-)��c(H2CO3)��c(OH-)��c(H+)��
��10?6.1��10?7.4��101.3�����ѪҺ��H2CO3��HCO3-��OH-��H+������Ũ���ɴ�С��ϵΪc(HCO3-)��c(H2CO3)��c(OH-)��c(H+)��
��6��ѪҺ�д���H2CO3 HCO3-+H+ƽ�⣬������ǿʱ��ѪҺ��������Ũ��������ƽ�������ƶ���������ǿʱ����������Ũ������ƽ�������ƶ�����˿���ά��ѪҺ��pH�ȶ���
HCO3-+H+ƽ�⣬������ǿʱ��ѪҺ��������Ũ��������ƽ�������ƶ���������ǿʱ����������Ũ������ƽ�������ƶ�����˿���ά��ѪҺ��pH�ȶ���
���㣺������������Է�Ӧ���ʺ�ƽ��״̬��Ӱ�졢���淴Ӧ�����Լ���Һ��pH���������Ũ�ȴ�С�Ƚ�

��6�֣�ijѧϰС����������ϡ����ķ�Ӧ�����С�̽��Ӱ�컯ѧ��Ӧ�������ء���ʵ�顣������±���
| ʵ�� ��� | ��������/g | ���� ��̬ | V(H2SO4) /mL | c(H2SO4) /mol��L-1 | ��Ӧǰ��Һ ���¶�/�� | ������ȫ�� ʧ��ʱ��/s |
| 1 | 0.10 | Ƭ״ | 50 | 0.8 | 20 | 200 |
| 2 | 0.10 | ��״ | 50 | 0.8 | 20 | 25 |
| 3 | 0.10 | Ƭ״ | 50 | 1.0 | 20 | 125 |
| 4 | 0.10 | Ƭ״ | 50 | 1.0 | 35 | 50 |
��1��ʵ��1��2 ������������ ���Է�Ӧ������Ӱ�졣
��2����������Ӧ��Ũ�ȶԷ�Ӧ���ʲ���Ӱ���ʵ������ �� ����ʵ����ţ���
��3����̽��ʵ���У�Ӱ�췴Ӧ���ʵ����ػ��������� ���������������ָ�Ӱ�����ص�ʵ��������������������ʵ����ţ���
��4������ʵ��3ʱ������ϡ������Ϊ50 mL 2.0 mol��L-1 ���ᣨ�����������䣩�����֣��ų����ݵ����ʣ��������Ա�����졣����Ϊ���ܵ�ԭ���� ���������¶ȶԷ�Ӧ���ʵ�Ӱ�죩
(14��)
�������ڹ�ҵ�������й㷺����;��
��1�����ڿ��淴Ӧ2SO2(g)��O2(g)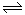 2SO3(g) ��H��0�������о�Ŀ�ĺ�ͼʾ�������
2SO3(g) ��H��0�������о�Ŀ�ĺ�ͼʾ�������
������ţ�
| ��� | A | B | C | D |
| Ŀ�� | ѹǿ��ƽ���Ӱ�� | �¶ȶ�ת���ʵ�Ӱ�� | ����O2Ũ�ȶ����ʵ�Ӱ�� | Ũ�ȶ�ƽ�ⳣ����Ӱ�� |
| ͼʾ | 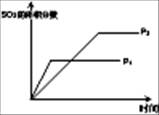 |  | 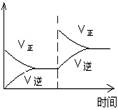 | 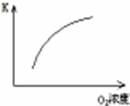 |
�ҡ����>����<����="��" ��
��3����2L�ļ������г���2molSO2��1molO2�����SO2��ƽ��ת�������¶ȵĹ�ϵ����ͼ��ʾ��
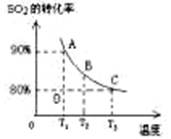
��.��T1�¶��£���Ӧ���е�״̬Dʱ��v�� v�������>����<����="��" ��
��.T3�¶��£�ƽ��ʱ��÷�Ӧ�ų�������ΪQ1������ͬ�¶���������������ͨ��2molSO2��1molO2�����´ﵽƽ�⣬��÷�Ӧ�ַų�����Q2 ��������˵������ȷ���� ��������ţ�
A����ͬ�¶�����ƽ��ʱ�����е�ѹǿ��ԭƽ��ʱ������
B��Q2һ������Q1
C����ƽ��ʱSO2��ת����һ������80%
��
��4���ڼ������г���һ������SO2��1.100molO2���ڴ��������¼��ȣ�����������ʵ�������0.315molʱ��Ӧ�ﵽƽ�⣬��ʱ�������ѹǿΪ��Ӧǰ��82.5%����SO2��ת����Ϊ ��
����5����������������Һ����SO2����ǡ�õõ���ʽ�Σ���֪����ʽ����Һ�������ԣ�����Һ�и�����Ũ���ɴ�С��˳��Ϊ ����������Ũ�ȷ��ű�ʾ��
����6��һ���¶��£���ˮ����SO2���壬���õ�pH=5��H2SO3��Һ������Һ��������������Ӻ�����������ӵ����ʵ���Ũ��֮��Ϊ ���� ������֪���¶���H2SO3�ĵ��볣����Ka1=1.0��10-2mol/L��Ka2=6.0��10-3mol/L��
��������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸�����ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)  CH3OH(g)��H2O(g)���÷�Ӧ�������仯��ͼ��ʾ��
CH3OH(g)��H2O(g)���÷�Ӧ�������仯��ͼ��ʾ��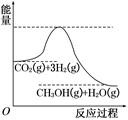
(1)������Ӧƽ�ⳣ��K�ı���ʽΪ ���¶Ƚ��ͣ�ƽ�ⳣ��K (����������䡱��С��)��
(2)�����Ϊ2 L���ܱ������У�����1 mol CO2��3 mol H2�����CO2�����ʵ�����ʱ��仯���±���ʾ���ӷ�Ӧ��ʼ��5 minĩ��������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(H2)�� ��
| t/min | 0 | 2 | 5 | 10 | 15 |
| n(CO2)/mol | 1 | 0.75 | 0.5 | 0.25 | 0.25 |
(3)����������ʹ������Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ����� (��д�����ĸ)
a����ʱ�����CH3OH����
b���ʵ������¶�
c�������������ݻ����䣬�ٳ���1 mol CO2��3 mol H2
d��ѡ���Ч����
 (NH4)2CO3(aq) ��H1
(NH4)2CO3(aq) ��H1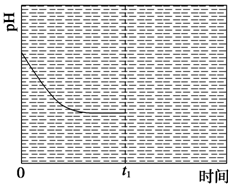
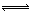 NaNO3��s��+ClNO��g�� K1 ?H < 0 ��I��
NaNO3��s��+ClNO��g�� K1 ?H < 0 ��I��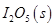 �����ڼ��CO����Ӧԭ��Ϊ��
�����ڼ��CO����Ӧԭ��Ϊ��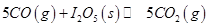
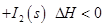 ����2L�ܱ������м�������
����2L�ܱ������м�������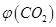 ��ʱ��ı仯����ͼ��ʾ��
��ʱ��ı仯����ͼ��ʾ��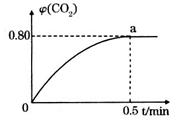
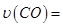 _____________��
_____________��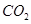 ���������
���������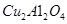 Ϊ���������Խ�
Ϊ���������Խ�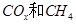 �Ļ������ֱ��ת��Ϊ���ᡣ
�Ļ������ֱ��ת��Ϊ���ᡣ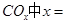 ______________��
______________��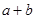 ________14(�>������<����=��)�����¶��´���ĵ��볣��K=__________(�ú�a��b��ʽ�ӱ�ʾ)��
________14(�>������<����=��)�����¶��´���ĵ��볣��K=__________(�ú�a��b��ʽ�ӱ�ʾ)��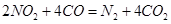 ���Դ�������β���������÷�Ӧ���Ϊԭ��أ�������Na2O������ʣ��������缫��ӦʽΪ________________________________��
���Դ�������β���������÷�Ӧ���Ϊԭ��أ�������Na2O������ʣ��������缫��ӦʽΪ________________________________�� xC��g��+2D��g��������5min��Ӧ�ﵽƽ��״̬�������ڵ�ѹǿ��С����֪D��ƽ����Ӧ����Ϊ0.2mol/��L?min��,����д���пհף�
xC��g��+2D��g��������5min��Ӧ�ﵽƽ��״̬�������ڵ�ѹǿ��С����֪D��ƽ����Ӧ����Ϊ0.2mol/��L?min��,����д���пհף�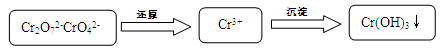
 Cr2O72- + H2O
Cr2O72- + H2O