��Ŀ����
����Ŀ���ߴ�������[��ѧʽ��Sr(NO3)2]���������źŵơ���ѧ�����ȡ�
��ҵ���������г���������ơ����ᱵ�����ʣ���������ƿ�����Ũ���ᣬ�������ȡ����ᱵ������Ũ���ᡣ�ᴿ�����ȵ�ʵ�鲽�����£�
��ȡ�����ʵ���������Ʒ�������м���ŨHNO3�ܽ⣬���衣
�ڹ��ˣ�����ŨHNO3ϴ��������
�۽���������ˮ�У����Թ�������ʹBa2+���������ú�����£�N2H4�����������ỹԭ������pH��7��8�����ˡ�
�ܽ���Һ���������pH��2��3������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӡ�
�ݽ��õ���Sr(NO3)2��2H2O������100 �������¸���õ��ߴ������ȡ�
��1���������ŨHNO3ϴ��������Ŀ����_________��
��2����������£�N2H4�����������ᣨCrO3����ԭΪCr3+��ͬʱ����һ����ɫ��ζ�����壬д���÷�Ӧ�����ӷ���ʽ_______________��
��3���¾��кܺõĻ�ԭ�ԣ���������������Ⱦ���ʿ����ڳ�ȥ��¯���豸��ˮ�е��ܽ����ȣ���ȥ100L��¯ˮ��������8g/L�����ܽ�������Ҫ�µ�����Ϊ_________��
��Sr(NO3)2�����ֽ⣬����Sr(NO2)2��O2����500 ��ʱSr(NO2)2��һ���ֽ�����SrO���������ȡһ��������Sr(NO2)2��Sr(NO3)2��Ʒ����������ȫ�ֽ⣬�õ�5.20 g SrO�����5.08 g������塣�������Ʒ��Sr(NO3)2������������д��������̣�_________����ȷ��0.01����
���𰸡� ��ȥ���ʼ��������ȵ��ܽ���ʧ 3N2H4 + 4CrO3+12H+=3N2��+ 4Cr3++12H2O 800g m(��Ʒ)= 5.20 g+5.08 g=10.28 g n(SrO)= ![]() =0.05 mol
=0.05 mol
n[Sr(NO3)2]��212 g��mol-1+ n[Sr(NO2)2]��180 g��mol-1=10.28 g
n[Sr(NO3)2]+ n[Sr(NO2)2] =0.05 mol
���:n[Sr(NO3)2] =0.04 mol (1��) n[Sr(NO2)2] =0.01 mol (1��)
w[Sr(NO3)2] = ![]() ��100%=82.49% (2 ��)
��100%=82.49% (2 ��)
����������1���������к�������ơ����ᱵ�����ʣ�������ƿ�����Ũ���ᣬ�������ȡ����ᱵ������Ũ���ᣬ����ȼ���Ũ���ᣬ�ܽ�����ƣ��ﵽ ��ȥ���ʼ��������ȵ��ܽ���ʧ��Ŀ����
��2���£�N2H4�����������ᣨCrO3����ԭΪCr3+��ͬʱ����N2����ϵ����غ㡢����غ㼰ԭ���غ�ô˷�Ӧ�����ӷ���ʽ3N2H4 + 4CrO3+12H+=3N2��+ 4Cr3++12H2O��
��3���³����ķ�Ӧ�����º���������Ϊ������������Ⱦ�������������ϳɵ�ˮ�����������غ㶨�ɿ�֪�����ﻹ�е������ٸ��ݹ۲취��ƽ�����Է���ʽ�ǣ�N2H4+O2=N2+2H2O����Ҫ�ܽ���������ʵ���Ϊ![]() =25mol������Ҫ�µ�����Ϊ25mol��32g/mol=800g��
=25mol������Ҫ�µ�����Ϊ25mol��32g/mol=800g��
��m����Ʒ��=5.20g+5.08g=10.28g��
n��SrO��=![]() =0.05mol��
=0.05mol��
n[Sr��NO3��2]��212gmol-1+n[Sr��NO2��2]��180gmol-1=10.28 g��
n[Sr��NO3��2]+n[Sr��NO2��2]=0.05mol��
��ã�n[Sr��NO3��2]=0.04mol��
n[Sr��NO2��2]=0.01mol��
��[Sr��NO3��2]= ![]() ��100%=82.49%��
��100%=82.49%��

 �Ķ��쳵ϵ�д�
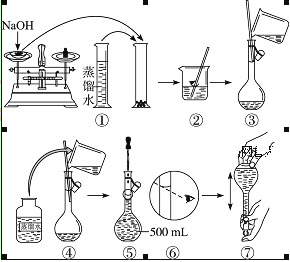
�Ķ��쳵ϵ�д�����Ŀ������400 mL 0.5 mol��L��1��NaOH��Һ���Իش��������⣺
��1�����㣺��ҪNaOH���������Ϊ______��
��2��ijѧ����������ƽ����һ��С�ձ�������������ǰ��������ڱ�ߵ���̶ȴ�����ƽ��ֹʱ���� ָ���ڷֶ��̵�ƫ��λ�ã���ʱ��ߵ����̽�______(��������������������)�ұߵ����̡���ʹ��ƽƽ�⣬�����еIJ���Ϊ_______���ٶ����ճƵ�С�ձ�������Ϊ______(����32.6 g������31.61 g��)��
��3�����Ʒ������������������裺
�� ��ʢ��NaOH���ձ��м���200 mL����ˮʹ���ܽ⣬����ȴ�����£�
�� ����������ƿ�м�����ˮ��Һ��ӽ��̶���1��2 cm����
�� ��NaOH��Һ�ز�����ע��500 mL����ƿ�У�
�� ���ձ��м�������������ˮ��С��ϴ��2��3�κ���������ƿ��
�� ���ý�ͷ�ιܼ�����ˮ���̶��ߣ��Ӹ�ҡ�ȡ�
�Խ����ϲ����ų��Ⱥ�˳��______��
��4��ijѧ��ʵ������NaOH��Һ��Ũ��Ϊ0.48 mol��L��1��ԭ�������______��
A��ʹ����ֽ�����������ƹ��� |
B������ƿ��ԭ��������������ˮ |
C���ܽ�NaOH���ձ�δ�����ϴ�� |
D����ͷ�ιܼ�ˮ����ʱ���ӿ̶� |
��5������������0.5 mol��L��1NaOH��Һ����ʾ��ͼ���д������(�����)______��