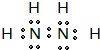��Ŀ����
���Ϊ��ʽ���ڱ���һ���֣����б�Ŵ�����Ӧ��Ԫ�أ�
��1��Ԫ�آڡ��ۡ��ܵĵ�һ��������С����˳����Ԫ�ط��ű�ʾ�� ��Ԫ�آ�̬ԭ�ӵ���Χ�����Ų�ʽ ��
��2��Ԫ�آ١��ڵ�һ�ֻ���������Ҫ�Ļ���ԭ�ϣ����Ѹû�����IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظû��������˵����ȷ���� ��
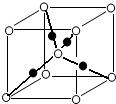
A������ԭ�Ӳ�������ͬһƽ���� B�����ڷǼ��Է���
C������4���Ҽ���1���м� D����ʯ���ѽ�������Ҫ�ɷ�
��3��Ԫ�آ���ݿ��γ�һ�ֵ���ɫ�Ĺ��廯����侧�������� ��д����������ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽ ��
��4���ڲⶨԪ�آ�������γɵ�һ�ֺ���ɫ���廯������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ���� ��
��5����25�桢101kPa�£���֪����̬�⻯������������ȫȼ�պ�ָ���ԭ״̬��ÿת��1mol e-����190.0kJ������̬�⻯��ȼ���ȡ�H= ��
| �� | ||||||||||||||||||
| �� | �� | �� | ||||||||||||||||
| �� | �� | |||||||||||||||||
| �� | ||||||||||||||||||
��2��Ԫ�آ١��ڵ�һ�ֻ���������Ҫ�Ļ���ԭ�ϣ����Ѹû�����IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظû��������˵����ȷ����
A������ԭ�Ӳ�������ͬһƽ���� B�����ڷǼ��Է���
C������4���Ҽ���1���м� D����ʯ���ѽ�������Ҫ�ɷ�
��3��Ԫ�آ���ݿ��γ�һ�ֵ���ɫ�Ĺ��廯����侧��������
��4���ڲⶨԪ�آ�������γɵ�һ�ֺ���ɫ���廯������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ����
��5����25�桢101kPa�£���֪����̬�⻯������������ȫȼ�պ�ָ���ԭ״̬��ÿת��1mol e-����190.0kJ������̬�⻯��ȼ���ȡ�H=
��������Ԫ�������ڱ���λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪSi����ΪNi��
��1��ͬ���ڣ���ԭ�����������һ�����ܳ��������ƣ���ԭ���Ų�����ȫ����������ȫ��ʱ�����ϵͣ���һ�����ܽϸߣ�
Niԭ�Ӻ��������Ϊ28������������ԭ����д��Χ�����Ų���
��2��Ԫ�آ١��ڵ�һ�ֻ�����IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����û�����ΪC2H4���ṹʽΪ ��Ϊƽ���ͽṹ������Ϊ�Գƽṹ�����ڷǽ����Է��ӣ�������ʯ���ѽ����ɣ�
��Ϊƽ���ͽṹ������Ϊ�Գƽṹ�����ڷǽ����Է��ӣ�������ʯ���ѽ����ɣ�
��3��Ԫ�آ���ݿ��γ�һ�ֵ���ɫ�Ĺ��廯����ΪNa2O2���������ӻ������ˮ��Ӧ����NaOH��������
��4��Ԫ�آ�������γɵ�һ�ֺ���ɫ���廯����ΪNO2������ƽ��2NO2?N2O4������ת��ΪN2O4���ⶨ��Է�������ƫ��
��5��1molSiH4��ȫȼ�����ɶ���������Һ̬ˮ��ת�Ƶ�����Ϊ8mol���ݴ˼���ų�������������ȷ����ȼ���ȣ�
��1��ͬ���ڣ���ԭ�����������һ�����ܳ��������ƣ���ԭ���Ų�����ȫ����������ȫ��ʱ�����ϵͣ���һ�����ܽϸߣ�
Niԭ�Ӻ��������Ϊ28������������ԭ����д��Χ�����Ų���
��2��Ԫ�آ١��ڵ�һ�ֻ�����IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����û�����ΪC2H4���ṹʽΪ
 ��Ϊƽ���ͽṹ������Ϊ�Գƽṹ�����ڷǽ����Է��ӣ�������ʯ���ѽ����ɣ�
��Ϊƽ���ͽṹ������Ϊ�Գƽṹ�����ڷǽ����Է��ӣ�������ʯ���ѽ����ɣ���3��Ԫ�آ���ݿ��γ�һ�ֵ���ɫ�Ĺ��廯����ΪNa2O2���������ӻ������ˮ��Ӧ����NaOH��������
��4��Ԫ�آ�������γɵ�һ�ֺ���ɫ���廯����ΪNO2������ƽ��2NO2?N2O4������ת��ΪN2O4���ⶨ��Է�������ƫ��
��5��1molSiH4��ȫȼ�����ɶ���������Һ̬ˮ��ת�Ƶ�����Ϊ8mol���ݴ˼���ų�������������ȷ����ȼ���ȣ�
����⣺��Ԫ�������ڱ���λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪSi����ΪNi��
��1��ͬ���ڣ���ԭ�����������һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ�����3�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܸ�����Ԫ�أ��ʵ�һ������N��O��C��
Niԭ�Ӻ��������Ϊ28�����������ԭ����֪������Χ�����Ų�Ϊ��3d84s2��
�ʴ�Ϊ��N��O��C��3d84s2��
��2��Ԫ�آ١��ڵ�һ�ֻ�����IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����û�����ΪC2H4��
A����ϩΪƽ��ṹ������ԭ�Ӷ���ͬһƽ���ڣ���A����
B����ϩ����Ϊƽ��Գƽṹ��������������������غϣ����ڷǼ��Է��ӣ���B��ȷ��
C����ϩ���ӽṹʽΪ �������к���5���Ҽ���1���м�����C����
�������к���5���Ҽ���1���м�����C����
D����ϩ��ʯ���ѽ�������Ҫ�ɷ֣���D��ȷ��
�ʴ�Ϊ��BD��
��3��Ԫ�آ���ݿ��γ�һ�ֵ���ɫ�Ĺ��廯����ΪNa2O2���������ӻ������ˮ��Ӧ����NaOH����������Ӧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ�����ӻ����2Na2O2+2H2O=4NaOH+O2����
��4��Ԫ�آ�������γɵ�һ�ֺ���ɫ���廯����ΪNO2������ƽ��2NO2?N2O4������ת��ΪN2O4���ⶨ��Է�������ƫ��
�ʴ�Ϊ������ƽ��2NO2?N2O4��NO2����ת��ΪN2O4��
��5����SiH4+2O2
SiO2+2H2O��ÿת��lmol����ʱ����190.0kJ���÷�Ӧ��SiԪ����-4�����ߵ�+4�ۣ���ת��8mol����ʱ�ų�190.0kJ��8=1520kJ����SiH4ȼ���ȡ�H=-1520kJ/mol��
�ʴ�Ϊ��-1520kJ/mol��
��1��ͬ���ڣ���ԭ�����������һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ�����3�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܸ�����Ԫ�أ��ʵ�һ������N��O��C��
Niԭ�Ӻ��������Ϊ28�����������ԭ����֪������Χ�����Ų�Ϊ��3d84s2��
�ʴ�Ϊ��N��O��C��3d84s2��
��2��Ԫ�آ١��ڵ�һ�ֻ�����IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־����û�����ΪC2H4��
A����ϩΪƽ��ṹ������ԭ�Ӷ���ͬһƽ���ڣ���A����
B����ϩ����Ϊƽ��Գƽṹ��������������������غϣ����ڷǼ��Է��ӣ���B��ȷ��
C����ϩ���ӽṹʽΪ
 �������к���5���Ҽ���1���м�����C����
�������к���5���Ҽ���1���м�����C����D����ϩ��ʯ���ѽ�������Ҫ�ɷ֣���D��ȷ��
�ʴ�Ϊ��BD��
��3��Ԫ�آ���ݿ��γ�һ�ֵ���ɫ�Ĺ��廯����ΪNa2O2���������ӻ������ˮ��Ӧ����NaOH����������Ӧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ�����ӻ����2Na2O2+2H2O=4NaOH+O2����
��4��Ԫ�آ�������γɵ�һ�ֺ���ɫ���廯����ΪNO2������ƽ��2NO2?N2O4������ת��ΪN2O4���ⶨ��Է�������ƫ��
�ʴ�Ϊ������ƽ��2NO2?N2O4��NO2����ת��ΪN2O4��
��5����SiH4+2O2
| ||
�ʴ�Ϊ��-1520kJ/mol��
���������⿼��Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã��漰Ԫ�ػ��������ʡ������ܡ���������Ų�����ѧ�������ӽṹ�����ʡ�ȼ���ȵȣ��Ѷ��еȣ�ע����������Ԫ�����ڱ��Ľṹ��ע���ȼ���ȵ����⣮

��ϰ��ϵ�д�
�����Ŀ