��Ŀ����
��ʵ��������ܶ�Ϊ1.84 g��cm-3����������Ϊ98.3%��Ũ��������250 mLŨ��Ϊ0.5 mol��L-1��ϡ���ᡣ��1����������Ũ����������___________________________mL��
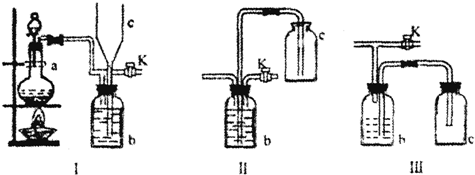
��2�����õ������������ǣ�ע����Ҫ�Ĺ��___________________________��
��3������ʱ�����в��裬��ȷ�IJ���˳����___________________________��
A.������ƿ�е���Һ�����Լ�ƿ�У������ϱ�ǩ
B.���ձ��е�������ҺС�Ľ��裬���ò���ȴ������
C.����ʽ�ζ�������ʢ����������ˮ���ձ���ȷ����(1)���м���õ�Ũ����
D.������ȴ�������ز�����С��ע������ƿ�У�ϴ���ձ���������2��3�Σ�ϴ��Һ��ע������ƿ��
E.���ý�ͷ�ιܼ�ˮ���̶���
F.�ӸǷ���ҡ��
G.����������ƿ�м�ˮ����̶���2��3 cm��
H.�������ƿ�Ƿ�©ˮ
��4������ʱ���ӿ̶���ʹ������Һ�����ʵ���Ũ��_____________���ƫ��ƫС�����䡱����
��1��5.8
��2��10 mL��Ͳ������ʽ�ζ��ܣ����ձ�����������250 mL����ƿ����ͷ�ι�
��3��HCBDGEFA
��4��ƫС
��������1������Ũ��������������Һ�е�����H2SO4��������ͬ���ݴ˿ɵã�
1.84 g��cm-3��V(H2SO4)��98.3%=250 mL��10-3 L��mL-1��0.5 mol��L-1��98 g��mol-1
���V(H2SO4)=5.8 mL��
��2������ʵ��IJ��������������Ҫ��������ע�������Ĺ��;��ȡ�
��4��c(H2SO4)= ![]() ��c(H2SO4)�Ƿ�ȷ�Լ�ƫ��ȡ����n(H2SO4)��V�������ݡ�����ʱ���ӿ̶���ʹ������Һ�����Vƫ����������Һ�����ʵ���Ũ��c(H2SO4)ƫС��
��c(H2SO4)�Ƿ�ȷ�Լ�ƫ��ȡ����n(H2SO4)��V�������ݡ�����ʱ���ӿ̶���ʹ������Һ�����Vƫ����������Һ�����ʵ���Ũ��c(H2SO4)ƫС��

 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�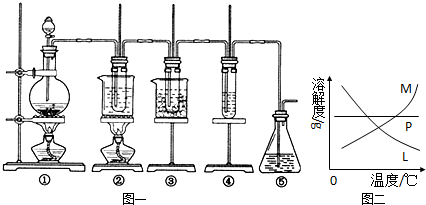
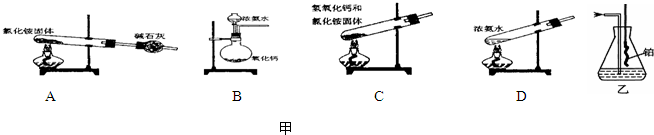 �ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��
�ڰ�����ʹʪ��ĺ�ɫ��ʯ����ֽ������ԭ���÷���ʽ��ʾ��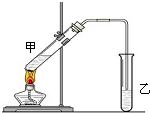 ��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺