��Ŀ����
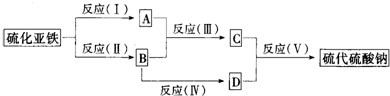
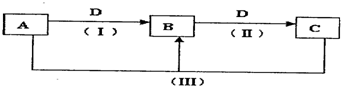
A��B��C��D������ѧ��ѧ���������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת���Ĺ�ϵ֪ͼ��ʾ��
��ش��������⣺
��1����DΪ���������;���Ľ������ʣ���������B����Һû�еõ�B���Σ���B�Ļ�ѧʽ����Ϊ______����д��һ�ּ��ɣ�
��2����ͨ�������A��B��C��D�������壬��B��DΪ��������Ҫ�ɷ֣���A�ĵ���ʽΪ______��B�ĽṹʽΪ______��
��3����DΪ�ȼҵ����Ҫ��Ʒ����Ӧ�������ӷ���ʽΪ______��
��4����A��B��C����Һ���Լ��ԣ�CΪ���Ƹ��ķ��ͷ۵���Ҫ�ɷ�֮һ��Ҳ����Ϊҽ��������θ�����֢��ҩ����
��25��ʱ��pH��Ϊ10��A��B����Һ�У���ˮ�����������������Ũ��֮��Ϊ______
��25��ʱ��0.1mol?L-1��A��B��C������Һ���ֱ���ˮϡ�Ͳ�ͬ�ı�������Һ��pH��ͬ����ϡ�ͺ���Һ�����ʵ���Ũ��������______��Һ�������ʵĻ�ѧʽ����
�۽������ʵ�����B��C����ˮ�γɻ����Һ����Һ�и�������Ũ���ɴ�С˳��Ϊ______��

��ش��������⣺
��1����DΪ���������;���Ľ������ʣ���������B����Һû�еõ�B���Σ���B�Ļ�ѧʽ����Ϊ______����д��һ�ּ��ɣ�
��2����ͨ�������A��B��C��D�������壬��B��DΪ��������Ҫ�ɷ֣���A�ĵ���ʽΪ______��B�ĽṹʽΪ______��
��3����DΪ�ȼҵ����Ҫ��Ʒ����Ӧ�������ӷ���ʽΪ______��
��4����A��B��C����Һ���Լ��ԣ�CΪ���Ƹ��ķ��ͷ۵���Ҫ�ɷ�֮һ��Ҳ����Ϊҽ��������θ�����֢��ҩ����
��25��ʱ��pH��Ϊ10��A��B����Һ�У���ˮ�����������������Ũ��֮��Ϊ______
��25��ʱ��0.1mol?L-1��A��B��C������Һ���ֱ���ˮϡ�Ͳ�ͬ�ı�������Һ��pH��ͬ����ϡ�ͺ���Һ�����ʵ���Ũ��������______��Һ�������ʵĻ�ѧʽ����
�۽������ʵ�����B��C����ˮ�γɻ����Һ����Һ�и�������Ũ���ɴ�С˳��Ϊ______��
��1����DΪ���������;���Ľ�������ΪFe����������B����Һû�еõ�B���Σ�˵�����ζ�Ӧ����Ϊ�ӷ����ᣬ��B�Ļ�ѧʽ����ΪFeCl3 ��Fe��NO3��3��ת����ϵ�ֱ�Ϊ��Cl2
FeCl3
FeCl2��HNO3
Fe��NO3��3
Fe��NO3��2���ʴ�Ϊ��FeCl3 ��Fe��NO3��3��
��2��B��DΪ��������Ҫ�ɷ֣���ΪN2��O2������A��B��C�ķ�Ӧ�ж���D�μӷ�Ӧ����DӦΪO2��BΪN2��ת����ϵΪ��
NH3
N2
��NO����AΪ NH3������ʽΪ
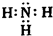
��BΪN2���ṹʽΪN��N���ʴ�Ϊ��
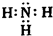
��N��N��
��3����DΪ�ȼҵ����Ҫ��Ʒ��ӦΪNaOH������NaOH������Ӧ�����ʷ�Ӧ��ת����ϵΪAl3+
Al��OH��3
AlO2-����Ӧ�������ӷ���ʽΪ
Al3++3AlO2-+6H2O=4Al��OH��3�����ʴ�Ϊ��Al3++3AlO2-+6H2O=4Al��OH��3����
��4������A��B��C����Һ���Լ��ԣ�CΪ���Ƹ��ķ��ͷ۵���Ҫ�ɷ�֮һ��Ҳ����Ϊҽ��������θ�����֢��ҩ������CΪNaHCO3��A��B��C��ת����ϵΪNaOH
Na2CO3
NaHCO3��25��ʱ��pH��Ϊ10��NaOH��Na2CO3����Һ�У���ˮ�����������������Ũ�ȷֱ�Ϊ��NaOH��c��OH-��=10-10mol/L��Na2CO3��c��OH-��=10-4mol/L��������ˮ�����������������Ũ��֮��Ϊ10-6��1��1��106���ʴ�Ϊ��10-6��1��1��106��
����ΪNaOH��ǿ�����Һ����ȫ���룬��Na2CO3��NaHCO3������ǿ���Σ�����ˮ�������¶�����ˮ���Լ��ԣ�Na2CO3��NaHCO3ˮ��ij̶�ֻ���ٲ��֣�����Na2CO3����NaHCO3ˮ��̶ȣ���PH��ͬʱ��NaOHŨ����С��NaHCO3Ũ����ʴ�Ϊ��NaHCO3��
�۽������ʵ�����Na2CO3��NaHCO3����ˮ�γɻ����Һ������CO32-����ˮ������HCO3-���ӣ���c��HCO3-����c��CO32-����Na2CO3��NaHCO3ˮ��ʼ��ԣ���c��OH-����c��H+����ˮ��ij̶�ֻ���ٲ��֣���c��CO32-����c��OH-��������˳��Ϊ��c��Na+ ����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+ ����c��HCO3-����c��CO32-����c��OH-����c��H+����
| Fe |
| Fe |
| Fe |
| Fe |
��2��B��DΪ��������Ҫ�ɷ֣���ΪN2��O2������A��B��C�ķ�Ӧ�ж���D�μӷ�Ӧ����DӦΪO2��BΪN2��ת����ϵΪ��
NH3
| O2 |
| O2 |
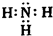
��BΪN2���ṹʽΪN��N���ʴ�Ϊ��
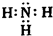
��N��N��
��3����DΪ�ȼҵ����Ҫ��Ʒ��ӦΪNaOH������NaOH������Ӧ�����ʷ�Ӧ��ת����ϵΪAl3+
| NaOH |
| NaOH |
Al3++3AlO2-+6H2O=4Al��OH��3�����ʴ�Ϊ��Al3++3AlO2-+6H2O=4Al��OH��3����
��4������A��B��C����Һ���Լ��ԣ�CΪ���Ƹ��ķ��ͷ۵���Ҫ�ɷ�֮һ��Ҳ����Ϊҽ��������θ�����֢��ҩ������CΪNaHCO3��A��B��C��ת����ϵΪNaOH
| CO2 |
| CO2 |
����ΪNaOH��ǿ�����Һ����ȫ���룬��Na2CO3��NaHCO3������ǿ���Σ�����ˮ�������¶�����ˮ���Լ��ԣ�Na2CO3��NaHCO3ˮ��ij̶�ֻ���ٲ��֣�����Na2CO3����NaHCO3ˮ��̶ȣ���PH��ͬʱ��NaOHŨ����С��NaHCO3Ũ����ʴ�Ϊ��NaHCO3��
�۽������ʵ�����Na2CO3��NaHCO3����ˮ�γɻ����Һ������CO32-����ˮ������HCO3-���ӣ���c��HCO3-����c��CO32-����Na2CO3��NaHCO3ˮ��ʼ��ԣ���c��OH-����c��H+����ˮ��ij̶�ֻ���ٲ��֣���c��CO32-����c��OH-��������˳��Ϊ��c��Na+ ����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+ ����c��HCO3-����c��CO32-����c��OH-����c��H+����

��ϰ��ϵ�д�
�����Ŀ
 A��B��C��D������ѧ��ѧ�г������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ��ʾ�����ַ�Ӧ�е�ˮ����ȥ����
A��B��C��D������ѧ��ѧ�г������ʣ�����A��B��C������ͬһ��Ԫ�أ���һ���������ת����ϵ��ͼ��ʾ�����ַ�Ӧ�е�ˮ����ȥ���� Fe��OH��3+3H+
Fe��OH��3+3H+