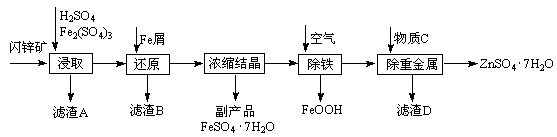
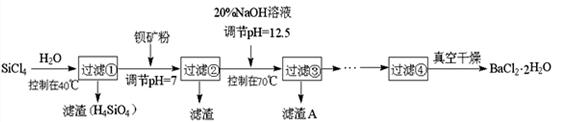
��Ŀ����
�廯��(CaBr2��2H2O)��һ�ְ�ɫ���壬������ˮ���к�ǿ����ʪ�ԣ��ǹ���ֽ��Ȫˮ����������Ҫ�ɷ֣���ҽҩ������������˥���ȵ�ҩ�Ҳ������ѧ�������ù�ҵ����ʯ����������Al3+��Fe3+�����ʣ��Ʊ��廯�Ƶ���Ҫ��������
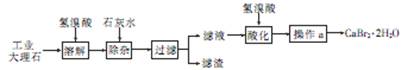
�ش���������
��1���ܽ�ʱ��������Ҫ��Ӧ�����ӷ���ʽΪ
��2�����Ӳ��������Һ��pHԼΪ8��0��Ŀ���� ��
��3����Һ���������ữ��Ŀ���� ������a��Ҫ���� �� ����
��4���Ƶõ��廯�ƾ������ͨ�����²���ⶨ�䴿�ȣ�
�ٳ�ȡ5��00g�廯�ƾ�����Ʒ�����ܽ⣻�۵�������Naa2CO3��Һ����ַ�Ӧ����ˣ��ܺ�ɡ���ȴ���ݳ��������õ�2�� 00 g̼��ƣ�����Ʒ�Ĵ���Ϊ
��5���廯�ƾ����������Ӻ����ӵļ���
�ٽ������廯�ƾ�������ˮ�����������ữ��AgNO3��Һ��ʵ������Ϊ ��������Ӧ�����ӷ���ʽΪ
�ڽ������廯�ƾ�������ˮ���μӲ�������Һ��ʵ������Ϊ ��������Ӧ�����ӷ���ʽΪ

�ش���������
��1���ܽ�ʱ��������Ҫ��Ӧ�����ӷ���ʽΪ
��2�����Ӳ��������Һ��pHԼΪ8��0��Ŀ���� ��
��3����Һ���������ữ��Ŀ���� ������a��Ҫ���� �� ����
��4���Ƶõ��廯�ƾ������ͨ�����²���ⶨ�䴿�ȣ�
�ٳ�ȡ5��00g�廯�ƾ�����Ʒ�����ܽ⣻�۵�������Naa2CO3��Һ����ַ�Ӧ����ˣ��ܺ�ɡ���ȴ���ݳ��������õ�2�� 00 g̼��ƣ�����Ʒ�Ĵ���Ϊ
��5���廯�ƾ����������Ӻ����ӵļ���
�ٽ������廯�ƾ�������ˮ�����������ữ��AgNO3��Һ��ʵ������Ϊ ��������Ӧ�����ӷ���ʽΪ
�ڽ������廯�ƾ�������ˮ���μӲ�������Һ��ʵ������Ϊ ��������Ӧ�����ӷ���ʽΪ
��1��CaCO3��2H��=Ca2����CO2����H2O��2�֣�
��2��ȷ��Al3����Fe3��������ȫ����ֹAl��OH��3�ܽ⣨��1�֣�
��3����ȥ������Ca��OH��2������Ũ������ȴ�ᾧ����1�֣�
��4��94��4%����0��944����2�֣�
��5���ٲ�������ɫ���ǣ�1�֣���Br����Ag��=AgBr����2�֣�
�ڲ�����ɫ������1�֣���Ca2++C2O42-=CaC2O4����2�֣�
��2��ȷ��Al3����Fe3��������ȫ����ֹAl��OH��3�ܽ⣨��1�֣�
��3����ȥ������Ca��OH��2������Ũ������ȴ�ᾧ����1�֣�
��4��94��4%����0��944����2�֣�
��5���ٲ�������ɫ���ǣ�1�֣���Br����Ag��=AgBr����2�֣�
�ڲ�����ɫ������1�֣���Ca2++C2O42-=CaC2O4����2�֣�
���������������ѧ����������Ҫע�⣺������ͷ����ȷ����������Ŀ�Ģ������̽�������Ϣ��ȷÿһ����ԭ����Ŀ�ĺͲ����۴���Ҫע��淶��ע�⻯ѧ�������дҪ��ʵ�������������������1������ʯ����Ҫ�ɷ�Ϊ̼��ƣ��������ᷴӦ�����廯�ơ�ˮ�Ͷ�����̼�����ӷ���ʽΪCaCO3��2H��=Ca2����CO2����H2O����2��������ҵ����ʯ�ijɷּ�����ͼ֪�����ӵ�Ŀ���dz�ȥAl3����Fe3���������������Ϊ���������������ǿ��ǿ�������Һ��pHԼΪ8��0��Ŀ����ȷ��Al3����Fe3��������ȫ����ֹAl��OH��3�ܽ⣻��3����Һ�к������������ƣ����������ữ��Ŀ���dz�ȥ������Ca(OH)2����Һ�еþ���IJ���Ϊ����Ũ������ȴ�ᾧ�����˵ȣ���4�����������غ㶨��֪��n(CaBr2)= n(CaCO3)=0.02mol���廯�ƾ���(CaBr2��2H2O)������Ϊ4.72g,��������Ϊ4.72/5.00��100%=94��4%����5�����������ữ����������Һ���������ӣ�ʵ������Ϊ��������ɫ���ǣ����ӷ���ʽΪBr����Ag��=AgBr�������ò�������Һ��������ӣ�����Ϊ������ɫ���������ӷ���ʽΪCa2++C2O42-=CaC2O4����

��ϰ��ϵ�д�
 ÿ�α���ϵ�д�
ÿ�α���ϵ�д�
�����Ŀ