��Ŀ����
�����Ƕ�ijˮ��Һ�������Ӽ���ķ����ͽ��ۣ�������ȷ���ǣ� ��
| A������NaOH��Һ�����ȣ�����ʹʪ�����ɫʯ����ֽ�������壬��ԭ��Һ��һ����NH4+ |
| B������������CaCl2��Һ�������˰�ɫ��������Һ��һ���д�����CO32�� |
| C���ýྻ�IJ�˿պȡ������Һ�����ڻ��������գ�����ɫ�ܲ����ܹ۲쵽�������ɫ������Һ��һ�����м����ӣ����ܺ��������� |
| D���ȼ����������Ὣ��Һ�ữ���ټ�AgNO3��Һ�������˰�ɫ��������Һ��һ�����д�����Cl�� |
C
���������A����������ˮ�Լ��ԣ�Ӧ��ʹʪ��ĺ�ɫʯ����ֽ����������B������������CaCl2��Һ�������˰�ɫ��������Һ�в�һ���д�����CO32���������ܺ����������������C���ýྻ�IJ�˿պȡ������Һ�����ڻ��������գ�����ɫ�ܲ����ܹ۲쵽�������ɫ������Һ��һ�����м����ӣ����ܺ��������ӣ���ȷ��D�������Ὣ��Һ�ữ�������������ӣ�����ȷ��ԭ��Һ��һ�����������ӣ�Ӧ�������ữ������

��ϰ��ϵ�д�
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
�����Ŀ
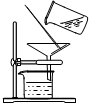
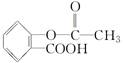 �ͱ�
�ͱ�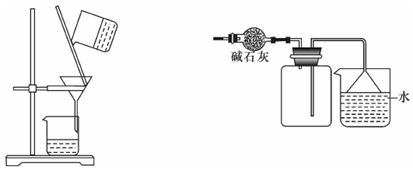

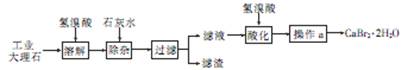

 Cu AlO2 �� ��ϵ��1ҲҪд��.
Cu AlO2 �� ��ϵ��1ҲҪд��.