��Ŀ����
14�������йؽ��������˵���в���ȷ���ǣ��������ٽ���������һ�֡����ͷ��ӡ�
�ڡ���������Ϊ����ԭ��������
�ۼ������ѻ��Ŀռ���������ͣ����������ѻ��Ŀռ����������
�ܽ����������Ժͱ����ԣ�ԭ����λ���ϸ�
�ݽ������ǽ��������Ӻ����ɵ���֮����ڵ�ǿ�ҵľ�������
���御����ȡ���ܶѻ���ʽ����ʹ���ñȽ��ȶ�
�߽���ͭ��þ����ABAB����ʽ�ѻ�
���ò��������β����ý��������۽���
���̬������ʱ���磬�۵���1 000�����ҵľ�������ǽ�������
��Al��Na��Mg���۵������ߣ�
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
���� �٢��������������ʵ���������ǡ����������ۡ��������۰ѽ���������Ϊ����ԭ�����������ļ۵����γɱ鲼���쾧��ġ�����������������ԭ�������У��Ӷ������н���ԭ��άϵ��һ��
�۽�������Ķѻ���ʽ�пռ������ʷֱ��ǣ�������52%����������68%����������Ϊ74%��
�ܽ�����û�з����Ժͱ����ԣ�
�ݴӻ��������������ʿ��������������Ӽ���ʵ�����ƣ������ڵ������ã�
����������������ͣ��������û�з����Ժͱ����Ե�����������γɾ���ʱ���ᾡ����ȡ���ܶѻ���ʽ����ʹ���ñȽ��ȶ���
��ͭ��ȡ����ABCABC���������ܶѻ���þ��ȡ����ABAB���������ܶѻ���
��������й�������Ϊ���ɵ����ܹ����տɼ��⣻
���������ں����磬�۵㲻�Ǻܸߣ��ų�ʯī�ȹ��壬ӦΪ�������壻
�����ԭ�ӵļ۵�����Խ�࣬ԭ�Ӱ뾶ԽС�����ɵ�������������Ӽ��������Խ�������۵�Խ�ߣ�
��� �⣺�ٰѽ���������Ϊ����ԭ�����������ļ۵����γɱ鲼���쾧��ġ�����������������ԭ�������У��Ӷ������н���ԭ��άϵ��һ�𣬽���������һ�֡����ͷ��ӡ����ʢ���ȷ��
�ڰѽ���������Ϊ����ԭ�����������ļ۵����γɱ鲼���쾧��ġ�����������������ԭ�������У��ʢ���ȷ��
�۽�������Ķѻ���ʽ�пռ������ʷֱ��ǣ�������52%����������68%����������Ϊ74%����˼������Ŀռ���������ͣ����������ռ���������ߣ��ʢ۴���
�ܽ������ǻ�ѧ����һ�֣���Ҫ�ڽ����д��ڣ������ɵ��Ӽ����гɾ���״�Ľ�������֮��ľ�����������϶��ɣ����ڵ��ӵ������˶���������û�й̶��ķ��ʢ���ȷ��
�ݽ������ǽ��������Ӻ����ɵ��������ִ����Ե�ɵ������ǿ�Ҿ������ã��Ȱ�������Ҳ�����ų����ã��ʢ���ȷ��
������û�з����Ժͱ����Ե�����������γɾ���ʱ�����御����ȡ���ܶѻ���ʽ��ʹ���ñȽ��ȶ����ʢ���ȷ��
��ͭ��ȡ����ABCABC���������ܶѻ���þ��ȡ����ABAB���������ܶѻ����ѻ���ʽ��ͬ���ʢߴ���
���ò��������Σ�����Ϊ�н������������й�������Ϊ���ɵ����ܹ����տɼ��⣬���ý���������֪ʶ���ͣ��ʢ����
���������ں����磬�۵㲻�Ǻܸߣ��ų�ʯī�ȹ��壬ӦΪ�������壬�ʢ���ȷ��
��Na Mg Al�ļ۵������������࣬ԭ�Ӱ뾶���μ�С���������������ǿ������Na Mg Al���۵��������ߣ��ʢ����
��ѡD��
���� ������Ҫ�����˽������塢����Ķѻ���ʽ��ԭ���Լ������۵���жϵȣ��ѶȲ���ע���Ӧ֪ʶ�����գ�

| A�� | ��һ�����ܣ�Li��Be��B | B�� | ������ۣ�O��N��C | ||
| C�� | �縺�ԣ�O��C��Si | D�� | �뾶��K+��Cl- |
| A�� | �٢ڢۢ� | B�� | ֻ�Тڢ� | C�� | �٢ڢ� | D�� | �ڢۢ� |
�ٵ�λʱ��������2n molA��ͬʱ����2n molC
�ڵ�λʱ��������n molB ��ͬʱ������2n molA
����A��C �����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ���ʵı�Ϊ1��1��״̬
�ܻ�������ѹǿ���ٸı��״̬
�ݻ��������ܶȲ��ٸı��״̬
��������ƽ����Է����������ٸı��״̬��
| A�� | �٢ܢ� | B�� | �ڢۢ� | C�� | �٢ܢݢ� | D�� | �٢ݢ� |
I������NaOH��Na2CO3���Һ�����ȣ�������Сʱ��
II���ų�ϴ�ӷ�Һ����ˮ��ϴ��¯������ϡ���������NaF��Һ�����ݣ�
III����ϴҺ�м���Na2SO3��Һ��
IV����ϴ��꣬��NaNO2��Һ�ۻ���¯��
��1���ڲ���I�У�
����NaOH��Ϊ���ܽ��ȥˮ���е�SiO2����Ӧ�Ļ�ѧ����ʽ��SiO2+2NaOH�TNa2SiO3+H2O��
����֪��20��ʱ�ܽ��/g
| CaCO3 | CaSO4 | Mg��OH��2 | MgCO3 |
| 1.4��10-3 | 2.55��10-2 | 9��10-4 | 1.1��10-2 |
��2���ڲ���II�У�
�ټ���ϡ���������NaF��Һ�ܳ�����ˮ����CaCO3��Mg��OH��2��Fe2O3��SiO2���ѧʽ����
����ϴ�����У��ܽ��Fe2O3����ٹ�¯��ʴ�������ӷ���ʽ������ԭ��2Fe3++Fe�T3Fe2+��
��3������III�У�����Na2SO3��Ŀ���ǽ�Fe3+��ԭ��Fe2+����ֹFe3+��ʴ��¯��
��4��������У��ۻ���Ĺ�¯����Ḳ��һ�����ܵ�Fe3O4����Ĥ��
����ɲ���ƽ�䷴Ӧ�����ӷ���ʽ��
��Fe+��NO${\;}_{2}^{-}$+��H2O�T��N2��+��Fe3O4+��
��������ۻ�Ч���ķ�����������ab������ţ���
a����¯���ϵμ�����CuSO4��Һ���۲���ɫ��ʧ��ʱ��
b����¯���ϵμ�����KSCN��Һ���۲��ɫ��ʧ��ʱ��
c����¯���ϵμ�ŨH2SO4���۲���Һ�����ػ�ɫ��ʱ��
d����¯���ϵμ�ŨHNO3���۲���ֺ���ɫ�����ʱ�䣮
| A�� | �ڻ�ѧ��Ӧ�У�ԭ���������ʱ�����������ı仯 | |
| B�� | ���ۺ���ά�صĻ�ѧʽ��Ϊ��C6H10O5��n���ʻ�Ϊͬ���칹�� | |
| C�� | ͨ����ѧ�仯���ԡ���ʯ�ɽ𡱣����ɽ����ת���ɽ��� | |
| D�� | �����������۰�a��b����;����ȫת����;��a��;��b���ĸ����NaOH ;��a��Al$��_{��ȼ}^{O_{2}}$Al2O3$\stackrel{NaOH��Һ}{��}$NaAlO2��;��b��Al$\stackrel{NaOH��Һ}{��}$NaAlO2 |
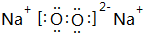 ��
��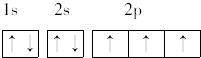 ����ԭ�Ӻ�����7���˶�״̬��ͬ�ĵ��ӣ�������ߵĵ���Ϊ2p����ϵĵ��ӣ������������Σ�
����ԭ�Ӻ�����7���˶�״̬��ͬ�ĵ��ӣ�������ߵĵ���Ϊ2p����ϵĵ��ӣ������������Σ�