��Ŀ����
17����д���пհף���1��������ĸ���ͬ���칹���У��˴Ź�������ֻ��һ�����շ�Ľṹ��ʽΪ
 ��
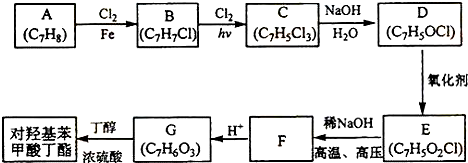
����2����A�뺬������ߵ���B����ͬϵ��ڹ�����1���A�������6���������ȫ��Ӧ��ͬ��ͬѹ������A�Ľṹʽ��
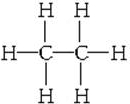
��3����C����Է���������72��C��һ���������������ͬ���칹�������ģ�C��������2-�����飨�������飩
��4��������D�����к�56�����ӣ�̼����Ԫ��������Ϊ12��1����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ
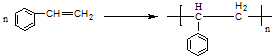 ��
����5��
 ���еĹ����ŵ�����Ϊ���ǻ���������
���еĹ����ŵ�����Ϊ���ǻ���������
���� ��1���˴Ź�������ֻ��һ�����շ壬˵�����л���������к���һ�ֵ�ЧHһ�֣�����л�����жԳƽṹ���ٽ�����������ͬ���칹��Ľṹ�����жϣ�
��2����������ߵ���BΪCH4��������1���A�������6���������ȫ��Ӧ�������к���6��Hԭ�ӣ�
��3��CnH2n+2����Է�������Ϊ72��n=5��C��һ���������������ͬ���칹�������ģ�����ڲ�ͬλ�õ�Hԭ����ࣻ
��4��������D�����к�56�����ӣ�̼����Ԫ��������Ϊ12��1��C��Hԭ�Ӹ���֮��Ϊ$\frac{12}{12}$��1=1��1����ΪC8H8��
��5�� ���з��ǻ���������
���з��ǻ���������
��� �⣺��1�������ͬ���칹���У������飺CH3CH2CH2CH2CH3�������� �������顢
�������顢 �����з�����ֻ��һ�ֵ�ЧHһ�ֵ�Ϊ�����飺
�����з�����ֻ��һ�ֵ�ЧHһ�ֵ�Ϊ�����飺 ��
��
�ʴ�Ϊ�� ��
��
��2����������ߵ���BΪCH4��������1���A�������6���������ȫ��Ӧ�������к���6��Hԭ�ӣ������Ϊͬϵ������Ϊ���飬�ṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3��CnH2n+2����Է�������Ϊ72��n=5������Ϊ���飬C��һ���������������ͬ���칹�������ģ�����ڲ�ͬλ�õ�Hԭ����࣬ӦΪCH3CH��CH3��CH2CH3����һ�������4�֣�����Ϊ2-�����飨�������飩���ʴ�Ϊ��2-�����飨�������飩��
��4��������D�����к�56�����ӣ�̼����Ԫ��������Ϊ12��1��C��Hԭ�Ӹ���֮��Ϊ$\frac{12}{12}$��1=1��1����ϵ�������֪����ΪC8H8�������Ӿ۷�ӦΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5���ɽṹ��ʽ��֪�л��� ���з��ǻ����������ʴ�Ϊ�����ǻ���������
���з��ǻ����������ʴ�Ϊ�����ǻ���������
���� ���⿼���л�����ƶϣ��漰ͬϵ�ͬ���칹�塢�Ӿ۷�Ӧ��ȡ����Ӧ����ȷ�л���Ľṹ�����ʼ��ɽ����Ŀ�ѶȲ���

| A�� | ����ױ� | B�� | �Զ��ױ� | C�� | ����ϩ | D�� | ��ϩ |
| A�� | ���Ż�ʱ����ĭ��������� | |
| B�� | �ռ���Һ�����ڴ���������ĥ�ڲ����Լ�ƿ�� | |
| C�� | Ũ���ᱣ������ɫϸ���Լ�ƿ�� | |
| D�� | ij��Һ����CCl4��CC14������ɫ��֤��ԭ��Һ�д���I- |
| A�� | ���۵⻯����Һ�ڿ����б�����4I+O2+2H2O�T4OH-+2I2 | |
| B�� | ��NaOH��Һ����������2OH-+2Cl2�T2Cl-+H2O | |
| C�� | ����CO2ͨ�뱽������Һ�У�2C6H5O-+CO2+H2O��2C6H5OH+CO32- | |
| D�� | �Ȼ�þ��Һ�백ˮ��Ӧ��Mg2++2OH-�TMg��OH��2�� |
| A�� | Mn | B�� | V | C�� | Cr | D�� | Cu |
| A�� | $\frac{1}{5}$mol | B�� | $\frac{2}{5}$ mol | C�� | $\frac{3}{5}$mol | D�� | $\frac{11}{5}$ mol |
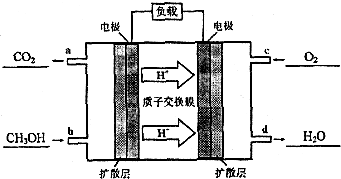 ��1���������²��0.1mol•L-1��ˮPH=11������¶��°�ˮ�ĵ���Ȧ�=1.0%
��1���������²��0.1mol•L-1��ˮPH=11������¶��°�ˮ�ĵ���Ȧ�=1.0%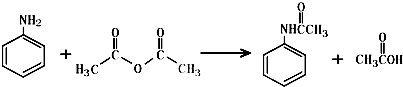
 ���÷�Ӧ������Ϊȡ����Ӧ��
���÷�Ӧ������Ϊȡ����Ӧ�� ��
��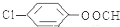 ��д�ṹ��ʽ����
��д�ṹ��ʽ����