��Ŀ����
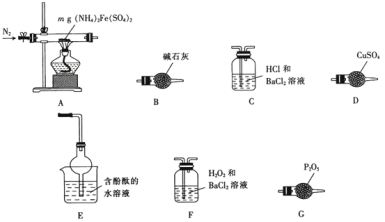
����Ŀ��I��ʵ��������ͼ��ʾ��װ����ȡ����������
(1)Ũ����������ǣ��� _______________���� ______________��
(2)�ұ�װ����ͨ�����ĵ���Ҫ����Һ���϶����ܲ�����Һ�У�Ŀ���Ƿ�ֹ��Һ�ĵ�������ɵ�����ԭ����____________��
(3)����õ����������ķ�����________________��������Ҫ�IJ���������___________��
(4)���ӵ���C2H518OHд�������������ķ���ʽ___________��
II��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣

(1)ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д���Ҵ��������Ļ�ѧ����ʽ____________���ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�����Ӧ��_______��Ӧ��
(2)��������ˮԡ���ò���ͬ����������_________���ҵ�������___________��
(3)��Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������__________������ƿ���ռ������������Ҫ�ɷ���____________��
(4)ͼ�б�������Ӧ��б��ԭ����______________��
���𰸡����� ��ˮ�� �ӷ������Ҵ�������������ˮ������ˮ����ѹǿ���������� ��Һ ��Һ©�����ձ� CH3COOH+CH3CH218OH![]() CH3CO18OCH2CH3+H2O 2CH3CH2OH+O2
CH3CO18OCH2CH3+H2O 2CH3CH2OH+O2![]() 2CH3CHO+2H2O ���� ���� ��ȴ ��ȩ���Ҵ���ˮ ���� ʹ�������Ҵ��������ڻ���
2CH3CHO+2H2O ���� ���� ��ȴ ��ȩ���Ҵ���ˮ ���� ʹ�������Ҵ��������ڻ���
��������
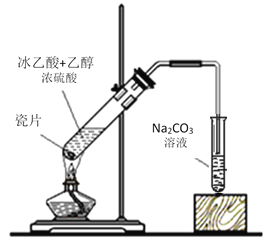
ʵ��������������ʱ���Ƚ�һ�������Ҵ������Թ��У�Ȼ�����Ũ���ᣬ����������ᣬ������Ӧ�Թ��ڼ������Ƭ���Է�Һ�屩�У��ڷ�Ӧ�����У��������ǻ��������ǻ��ϵ���ԭ�ӣ��������������л������ᡢ�Ҵ��������ñ���̼������Һ���ӣ�Ϊ���������������ܿ�Ӧλ��Һ���ϡ��ڼ�װ���У������ȣ��Ҵ�����������Ļ��������Ӳ�ʲ������У���Ӳ�ʲ������У�Cu����O2��Ӧ����CuO��CuO�ٱ��Ҵ���ԭΪCu���Ҵ�������Ϊ��ȩ�����ɵ���ȩ����װ���ھ�������ΪҺ�壬�����еĵ������뼯��ƿ�ڡ�
I��(1)�������Ҵ�����������Ӧ����Ӧ���ʺ�������������������Ũ����������ǣ��ٴ���������ˮ������Ϊ����������ˮ����
(2)��Ӧ�����������У�������Ҵ���������ˮ�������ұ�װ����ͨ�����ĵ���Ҫ����Һ���϶����ܲ�����Һ�У�Ŀ���Ƿ�ֹ��Һ�ĵ�������ɵ�����ԭ���ǻӷ������Ҵ�������������ˮ������ˮ����ѹǿ��������������Ϊ���ӷ������Ҵ�������������ˮ������ˮ����ѹǿ������������
(3)�������������ڱ���̼������Һ�����ܶȱȱ���̼������ҺС�����Է���õ����������ķ����Ƿ�Һ��������Ҫ�IJ��������Ƿ�Һ©�����ձ�����Ϊ����Һ����Һ©�����ձ���
(4)���ӵ���C2H518OH�����������ǻ�������Ĺ��ɣ������������ķ���ʽΪCH3COOH+CH3CH218OH![]() CH3CO18OCH2CH3+H2O����Ϊ��CH3COOH+CH3CH218OH
CH3CO18OCH2CH3+H2O������CH3COOH+CH3CH218OH![]() CH3CO18OCH2CH3+H2O��
CH3CO18OCH2CH3+H2O��
II��(1)�Ҵ�������ʱ��ת��Ϊ��ȩ��ˮ����ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O���ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�����Ӧ�Ƿ��ȷ�Ӧ����Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O���ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�����Ӧ�Ƿ��ȷ�Ӧ����Ϊ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O�����ȣ�
2CH3CHO+2H2O�����ȣ�
(2)�����Ϸ�����֪����������ˮԡ���ò���ͬ���������Ǽ��ȣ��ҵ���������ȴ����Ϊ�����ȣ���ȴ��
(3)��Ӧ����һ��ʱ���Ӧ���Pδ��Ӧ���Ҵ�������������������Թ�a�У���������ȩ���Ҵ���ˮ�������е�O2�����ĺ�N2���뼯��ƿ�У������ռ������������Ҫ�ɷ��ǵ�������Ϊ����ȩ���Ҵ���ˮ��������
(4)Ϊ��߷�Ӧ���ת���ʺ������ʣ�Ӧ��������������������ͼ�б�������Ӧ��б��ԭ����ʹ�������Ҵ��������ڻ�������Ϊ��ʹ�������Ҵ��������ڻ�����