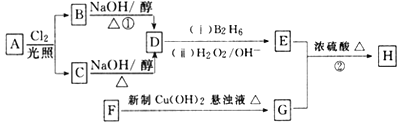
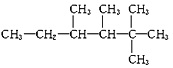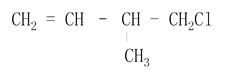��Ŀ����
����Ŀ��2002��ŵ������ѧ������ߵĹ���֮һ�Ƿ����˶��л�����ӽ��нṹ�������������䷽�����ü������ģ�10��9g��������ͨ�������ǵ����ӻ���ʹ��Ʒ���Ӵ������ӻ��������������ѳɸ�С�����ӡ���C2H6���ӻ���ɵõ�C2H6����C2H5����C2H4��������Ȼ��ⶨ���ʺɱȡ�ij�л�����Ʒ���ʺɱ�����ͼ��ʾ���������Ӿ���һ����λ����ɣ��ź�ǿ��������ӵĶ����йأ�������л���������� ��


A. �״� B. ���� C. ���� D. ��ϩ
���𰸡�C
��������������Ŀ�������������������������ӻ�ʱ���ɵĶ��Ǵ�һ����λ������ɣ������ʺɱȾ��Ǹ�������Է�����������˸��л������Է�������Ϊ16�����Ը��л���Ϊ���飬�ʱ�����ȷ��ΪC��

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�����Ŀ���о�+6�۸��β�ͬ��������������ʽ�������ԣ�ijС��ͬѧ��������ʵ�飺

��֪��Cr2O72- (��ɫ)+H2O![]() 2CrO42-(��ɫ)+2H+ ��H= +13.8 kJ/mol��+6�۸�����һ�������¿ɱ���ԭΪCr3+��Cr3+��ˮ��Һ��Ϊ��ɫ��
2CrO42-(��ɫ)+2H+ ��H= +13.8 kJ/mol��+6�۸�����һ�������¿ɱ���ԭΪCr3+��Cr3+��ˮ��Һ��Ϊ��ɫ��
��1���Թ�c��b�Աȣ��Ʋ��Թ�c��������________��
��2���Թ�a��b�Աȣ�a����Һ��ɫ�������Ϊ�¶�Ҳ��Ӱ��ƽ����ƶ�����ɫ���һ����c(H+)����Ӱ��Ľ��������Ϊ��ɫ����һ����c(H+)�����ƽ���Ӱ�졣����Ϊ�Ƿ���Ҫ�����ʵ��֤����____�����ǡ�����������_________________________________��
��3���Ա��Թ�a��b��c��ʵ�����õ��Ľ�����________________��
��4���Թ�c�����μ�KI��Һ������ϡH2SO4��������ͼ��ʵ�����ó��Ľ�����_______��д���˹�����������ԭ��Ӧ�����ӷ���ʽ________________��
��5��С��ͬѧ�õ�ⷨ������Cr2O72-��ˮ��̽����ͬ���ضԺ�Cr2O72-��ˮ������Ӱ�죬������±���ʾ��Cr2O72-����ʼŨ�ȣ��������ѹ�����ʱ�����ͬ����
ʵ�� | �� | �� | �� | �� |
�Ƿ����Fe2(SO4)3 | �� | �� | ����5g | �� |
�Ƿ����H2SO4 | �� | ����1mL | ����1mL | ����1mL |
�缫���� | ����������Ϊʯī | ����������Ϊʯī | ����������Ϊʯī | ����Ϊʯī������Ϊ�� |
Cr2O72-��ȥ����/% | 0.922 | 12.7 | 20.8 | 57.3 |
��ʵ������Cr2O72-�ŵ�ĵ缫��Ӧʽ��________________��
��ʵ����