��Ŀ����
��2010?����һģ��ͼ��ʾ��Ԫ�����ڱ���1��20���Ҳ�ͬ����Ԫ����ɵĵ��ʼ�������֮��ת����ϵ�������е�ˮ����ȥ��������AΪ���ʣ���ʵ�����У����ù���B����C������ȡ����F��F��G�����Ԫ����ͬ��G��I����������������ͬ����ش�

��1��д��B��G�Ļ�ѧʽB
��2��д��C�ĵ���ʽ
 ����Ӧ�ܵ�ʵ������Ϊ
����Ӧ�ܵ�ʵ������Ϊ
��3�������Ư�����õ�D��Һ����I��ϡ��Һ��д����Ӧ�����ӷ���ʽ��
��4��д����Ӧ�۵Ļ�ѧ����ʽ

��1��д��B��G�Ļ�ѧʽB
Ca��OH��2
Ca��OH��2
��GN2H4
N2H4
����2��д��C�ĵ���ʽ


����
����
��д��F��һ�־�����;�Ƶ��ʣ��������ᡢ�ƴ������Σ���ԭ�ϻ��������
�Ƶ��ʣ��������ᡢ�ƴ������Σ���ԭ�ϻ��������
����3�������Ư�����õ�D��Һ����I��ϡ��Һ��д����Ӧ�����ӷ���ʽ��
ClO-+H+=HClO
ClO-+H+=HClO
����D����Һ����I��Ũ��Һ��A���ɣ��䷴Ӧ�����ӷ���ʽΪ��ClO-+Cl-+2H+=Cl2��+H2O
ClO-+Cl-+2H+=Cl2��+H2O
����4��д����Ӧ�۵Ļ�ѧ����ʽ
2NH3+Ca��ClO��2=N2H4+CaCl2+H2O
2NH3+Ca��ClO��2=N2H4+CaCl2+H2O
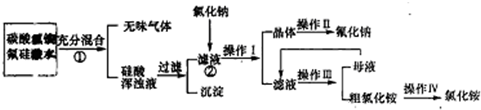
�������������ͻ�ƿ�Ϊ����ʵ�����У����ù���B����C������ȡ����F��ӦΪNH4Cl��Ca��OH��2��Ӧ���ɰ����ķ�Ӧ����FΪNH3��EΪCaCl2������F����I����C����CΪNH4Cl��BΪCa��OH��2��IΪHCl������F��G�����Ԫ����ͬ��G��I��ΪHCl������18�����ӣ�����������������ͬ���жϣ�GΪN2H4�������У�3�������Ư�����õ�D��Һ����֪DΪCa��ClO��2����AΪCl2���Դ����ش����и����⣮
����⣺��1����ʵ�����У����ù���B����C������ȡ����F��ӦΪNH4Cl��Ca��OH��2��Ӧ���ɰ����ķ�Ӧ����FΪNH3��EΪCaCl2������F����I����C����CΪNH4Cl��BΪCa��OH��2��IΪHCl������F��G�����Ԫ����ͬ��G��I��ΪHCl������18�����ӣ�����������������ͬ���жϣ�GΪN2H4�������У�3�������Ư�����õ�D��Һ����֪DΪCa��ClO��2����AΪCl2��
�ʴ�Ϊ��Ca��OH��2��N2H4��
��2��CΪNH4Cl��Ϊ���ӻ���������ʽΪ ����Ӧ��Ϊ��NH3+HCl�TNH4Cl���а������ɵ�����NH4Cl�������Ƶ��ʣ��ʴ�Ϊ��
����Ӧ��Ϊ��NH3+HCl�TNH4Cl���а������ɵ�����NH4Cl�������Ƶ��ʣ��ʴ�Ϊ�� �����̣��Ƶ��ʣ��������ᡢ�ƴ������Σ���ԭ�ϻ����������
�����̣��Ƶ��ʣ��������ᡢ�ƴ������Σ���ԭ�ϻ����������
��3��HClOΪ������ʣ���Ca��ClO��2��Һ�м���������HClO���ɣ�ClO-�����������¾���ǿ�����ԣ���Cl-��Ӧ����Cl2���ʴ�Ϊ��ClO-+H+=HClO��ClO-+Cl-+2H+=Cl2��+H2O��
��4�����ݷ�Ӧ����������������غ㶨�ɿ�д����Ӧ�۵Ļ�ѧ����ʽΪ2NH3+Ca��ClO��2=N2H4+CaCl2+H2O��
�ʴ�Ϊ��2NH3+Ca��ClO��2=N2H4+CaCl2+H2O��
�ʴ�Ϊ��Ca��OH��2��N2H4��
��2��CΪNH4Cl��Ϊ���ӻ���������ʽΪ
 ����Ӧ��Ϊ��NH3+HCl�TNH4Cl���а������ɵ�����NH4Cl�������Ƶ��ʣ��ʴ�Ϊ��
����Ӧ��Ϊ��NH3+HCl�TNH4Cl���а������ɵ�����NH4Cl�������Ƶ��ʣ��ʴ�Ϊ�� �����̣��Ƶ��ʣ��������ᡢ�ƴ������Σ���ԭ�ϻ����������
�����̣��Ƶ��ʣ��������ᡢ�ƴ������Σ���ԭ�ϻ������������3��HClOΪ������ʣ���Ca��ClO��2��Һ�м���������HClO���ɣ�ClO-�����������¾���ǿ�����ԣ���Cl-��Ӧ����Cl2���ʴ�Ϊ��ClO-+H+=HClO��ClO-+Cl-+2H+=Cl2��+H2O��
��4�����ݷ�Ӧ����������������غ㶨�ɿ�д����Ӧ�۵Ļ�ѧ����ʽΪ2NH3+Ca��ClO��2=N2H4+CaCl2+H2O��
�ʴ�Ϊ��2NH3+Ca��ClO��2=N2H4+CaCl2+H2O��
���������⿼��Ԫ�ػ�������ƶϣ�����ע��ץס��Ŀ�е���ɡ��ṹ�����ʵȹؼ�������Ϊ�����ͻ�ƿڣ������Ϊ�ۺϣ�����һ���Ѷȣ�ע��Ԫ�ػ��������֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ
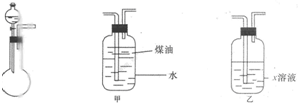 ��2010?������ģ����һ��ѧ��ƷNa2SO3�����ܺ���NaCl��Na2SO4��KNO3��K2CO2��K2SO4�е�һ�ֻ������ʣ�ijʵ��С������ͼ�ṩ��װ��ȷ������Ʒ�ijɷּ�Na2SO3�������������ƴ���Ʒ6.30g������6.0mol?L-1��������������������ɫ����560mL����״���������ݳ���������Һ�м����Թ�����BaCl2��Һ���õ���ɫ����9.32g������ɫ�겣���۲죬��Һ����ɫ��Ӧ����ɫ����ش��������⣺
��2010?������ģ����һ��ѧ��ƷNa2SO3�����ܺ���NaCl��Na2SO4��KNO3��K2CO2��K2SO4�е�һ�ֻ������ʣ�ijʵ��С������ͼ�ṩ��װ��ȷ������Ʒ�ijɷּ�Na2SO3�������������ƴ���Ʒ6.30g������6.0mol?L-1��������������������ɫ����560mL����״���������ݳ���������Һ�м����Թ�����BaCl2��Һ���õ���ɫ����9.32g������ɫ�겣���۲죬��Һ����ɫ��Ӧ����ɫ����ش��������⣺