��Ŀ����
 ��2010?������ģ����һ��ѧ��ƷNa2SO3�����ܺ���NaCl��Na2SO4��KNO3��K2CO2��K2SO4�е�һ�ֻ������ʣ�ijʵ��С������ͼ�ṩ��װ��ȷ������Ʒ�ijɷּ�Na2SO3�������������ƴ���Ʒ6.30g������6.0mol?L-1��������������������ɫ����560mL����״���������ݳ���������Һ�м����Թ�����BaCl2��Һ���õ���ɫ����9.32g������ɫ�겣���۲죬��Һ����ɫ��Ӧ����ɫ����ش��������⣺
��2010?������ģ����һ��ѧ��ƷNa2SO3�����ܺ���NaCl��Na2SO4��KNO3��K2CO2��K2SO4�е�һ�ֻ������ʣ�ijʵ��С������ͼ�ṩ��װ��ȷ������Ʒ�ijɷּ�Na2SO3�������������ƴ���Ʒ6.30g������6.0mol?L-1��������������������ɫ����560mL����״���������ݳ���������Һ�м����Թ�����BaCl2��Һ���õ���ɫ����9.32g������ɫ�겣���۲죬��Һ����ɫ��Ӧ����ɫ����ش��������⣺��1����ͼ��ҺX��
ŨH2SO4
ŨH2SO4
����ͼú�͵���������ֹSO2��ˮ�Ӵ�
��ֹSO2��ˮ�Ӵ�
����2����ʵ���м���������Һ�����Ϊ5.00mL������Ʒ��Na2SO3������������
50%
50%
��д���й����ӷ���ʽSO32-+2H+=SO2��+H2O��Ba2++SO42-=BaSO4��
SO32-+2H+=SO2��+H2O��Ba2++SO42-=BaSO4��
����3��������ṩ��ʵ�����������ʵ�飨ʵ��������ѡ�������ȷ������������������������裺
��Ӧװ���������º��������ƶ���Ͳ��ʹ��Ͳ���ƿ��Һ�汣��ˮƽ��Ȼ���ȡ���
��Ӧװ���������º��������ƶ���Ͳ��ʹ��Ͳ���ƿ��Һ�汣��ˮƽ��Ȼ���ȡ���
����4��˵��һ�����������Ƶ����ݣ�
�������������ʵ���Ϊ0.04mol����������������ʵ���Ϊ0.03mol�����Ա���������
�������������ʵ���Ϊ0.04mol����������������ʵ���Ϊ0.03mol�����Ա���������
����������������Ʒ6.30g������6.0mol?L-1��������������������ɫ����560mL����״����������ɫ�겣���۲죬��Һ����ɫ��Ӧ����ɫ��˵�������ӣ�֤���������һ����KNO3��K2CO2��K2SO4��������ɫ����560mL����״�����Ƕ����������ʵ���Ϊ0.025mol�����ݳ���������Һ�м����Թ�����BaCl2��Һ���õ���ɫ����9.32g���ж�Ϊ���ᱵ�������ʵ���=
=0.04mol��
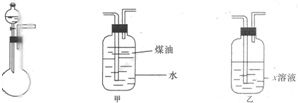
��1����ͼ��ϴ��ƿ����ȥ�������������е�ˮ��������ͼ������ˮ�����ⶨ��������������װ�ã�����ú���Ƿ�ֹ���������ˮ�Ӵ���Ӧ��
��2�������������ʵ������������������ʵ�������õ�������������Ӧ�����е����ӷ���ʽΪ����������Ӻ������ӷ�Ӧ���ɶ��������ˮ�����뱵���Ӻ���������ӽ���������ᱵ������
��3����ˮ����װ�ò�����������������õ�ԭ���������ų�Һ�������Ͳ�ж���Һ��������Ϊ��������������������ǣ���ȴ�����£���Ͳ���ƿҺ����ƽ�����������
��4������������������ʵ������㣬�������������غ�����жϣ�
| 9.32g |
| 233g/mol |
��1����ͼ��ϴ��ƿ����ȥ�������������е�ˮ��������ͼ������ˮ�����ⶨ��������������װ�ã�����ú���Ƿ�ֹ���������ˮ�Ӵ���Ӧ��
��2�������������ʵ������������������ʵ�������õ�������������Ӧ�����е����ӷ���ʽΪ����������Ӻ������ӷ�Ӧ���ɶ��������ˮ�����뱵���Ӻ���������ӽ���������ᱵ������
��3����ˮ����װ�ò�����������������õ�ԭ���������ų�Һ�������Ͳ�ж���Һ��������Ϊ��������������������ǣ���ȴ�����£���Ͳ���ƿҺ����ƽ�����������
��4������������������ʵ������㣬�������������غ�����жϣ�
����⣺��1����ͼ��ϴ��ƿ����ȥ�������������е�ˮ��������ͼ������ˮ�����ⶨ��������������װ�ã�����ú���Ƿ�ֹ���������ˮ�Ӵ���Ӧ��X��Һ��ŨH2SO4�������Ƿ�ֹSO2��ˮ�Ӵ���
�ʴ�Ϊ��ŨH2SO4����ֹSO2��ˮ�Ӵ���
��2������6.0mol?L-1����������������ʵ���м���������Һ�����Ϊ5.00mL��������ɫ����560mL����״���������������ʵ���Ϊ0.025mol�����������Ƶ����ʵ���Ϊ0.025mol����������=
��100%=50%��
�ʴ�Ϊ��50%��SO32-+2H+=SO2��+H2O��Ba2++SO42-=BaSO4����
��3����ˮ����װ�ò�����������������õ�ԭ���������ų�Һ�������Ͳ�ж���Һ��������Ϊ��������������������ǣ���ȴ�����£���Ͳ���ƿҺ����ƽ�����������
�ʴ�Ϊ����Ӧװ���������º��������ƶ���Ͳ��ʹ��Ͳ���ƿ��Һ�汣��ˮƽ��Ȼ���ȡ�����
��4���õ���ɫ����9.32g���ж�Ϊ���ᱵ�������ʵ���=
=0.04mol��������������ʵ���Ϊ0.03mol��˵��һ�����������ƣ�
�ʴ�Ϊ���������������ʵ���Ϊ0.04mol����������������ʵ���Ϊ0.03mol�����Ա��������ƣ�
�ʴ�Ϊ��ŨH2SO4����ֹSO2��ˮ�Ӵ���
��2������6.0mol?L-1����������������ʵ���м���������Һ�����Ϊ5.00mL��������ɫ����560mL����״���������������ʵ���Ϊ0.025mol�����������Ƶ����ʵ���Ϊ0.025mol����������=
| 0.025mol��126g/mol |
| 6.3g |
�ʴ�Ϊ��50%��SO32-+2H+=SO2��+H2O��Ba2++SO42-=BaSO4����
��3����ˮ����װ�ò�����������������õ�ԭ���������ų�Һ�������Ͳ�ж���Һ��������Ϊ��������������������ǣ���ȴ�����£���Ͳ���ƿҺ����ƽ�����������
�ʴ�Ϊ����Ӧװ���������º��������ƶ���Ͳ��ʹ��Ͳ���ƿ��Һ�汣��ˮƽ��Ȼ���ȡ�����
��4���õ���ɫ����9.32g���ж�Ϊ���ᱵ�������ʵ���=
| 9.32g |
| 233g/mol |
�ʴ�Ϊ���������������ʵ���Ϊ0.04mol����������������ʵ���Ϊ0.03mol�����Ա��������ƣ�
���������⿼�����������ʵ�̽����ʵ������жϣ�ʵ����Ƶ����ݺͲ��裬�������ʵ����ʺ�ʵ����������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ