��Ŀ����
����Ŀ����һ�ֿ�����ѩ�����Σ�����Ҫ�ɷֵĻ�ѧʽΪXY2 �� X��Y��Ϊ���ڱ�ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1mol XY2����54mol���ӡ�
��1������ѩ���Ļ�ѧʽ��_________���õ���ʽ��ʾXY2���γɹ���__________________��
��2������Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D��Y���ڣ� ��D�����ӽṹʾ��ͼ��_________��D��E���γ�һ��������CO2����ԭ�ӷ��ӣ���ÿ��ԭ�Ӿ��ﵽ��8e�ȶ��ṹ���÷��ӵĻ�ѧ������Ϊ_________������Ӽ������ۼ�������
��3���û�ѧʽ�ش�
��D��Y���⻯���ȶ���________��________��
��D��Y������������Ӧˮ��������________��________��
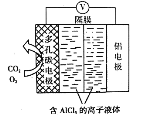
��4��ij��ȤС����Ƶļ���ԭ���װ������ͼ��ʾ���ش��������⣺

��пƬΪ_____�����������������
�ڵ��������������_________________����缫��ӦʽΪ_____________________��
�۵�����_______����������______���пƬ����̼��������
���𰸡�CaCl2 ![]()
 ���ۼ� HCl H2S HClO4 H2SO4 �� �����ݲ��� 2H+ + 2e- = H2�� ̼�� пƬ
���ۼ� HCl H2S HClO4 H2SO4 �� �����ݲ��� 2H+ + 2e- = H2�� ̼�� пƬ
��������
X��Y������ʵĻ�ѧʽΪXY2����Ϊ���ӻ�������XΪ��2�ۣ�YΪ��1�ۣ���Y�ĵ�����Ϊa����X�ĵ�����Ϊa��3������2a��a��3=54��a=17��YΪCl��XΪCa��D��Cl���ڣ�D��Eԭ�ӵ�����������������Ӳ�����2����DΪS��EΪC��Ȼ����з�����
X��Y������ʵĻ�ѧʽΪXY2����Ϊ���ӻ�������XΪ��2�ۣ�YΪ��1�ۣ���Y�ĵ�����Ϊa����X�ĵ�����Ϊa��3������2a��a��3=54��a=17��YΪCl��XΪCa��D��Cl���ڣ�D��Eԭ�ӵ�����������������Ӳ�����2����DΪS��EΪC��
��1��������������������ѩ���Ļ�ѧʽΪCaCl2��CaCl2Ϊ���ӻ�����õ���ʽ��ʾ���γɹ���Ϊ![]() ��
��
��2��DΪS��������ΪS2�����ṹʾ��ͼΪ�� ��S��C�γ�һ��������CO2����ԭ�ӷ��ӣ�����ѧʽΪCS2�����ڹ��ۻ�������й��ۼ���
��S��C�γ�һ��������CO2����ԭ�ӷ��ӣ�����ѧʽΪCS2�����ڹ��ۻ�������й��ۼ���
��3����ͬ���ڴ������ҷǽ�������ǿ�����⻯����ȶ�����ǿ����HCl���ȶ���ǿ��H2S��
�ڷǽ�����Խǿ��������������Ӧˮ���������Խǿ����HClO4������ǿ��H2SO4��
��4���ٸ���ԭ��ع���������пƬΪ������
��̼��Ϊ�����������ϵĵ缫��ӦʽΪ2H����2e��=H2���������ϵ������������ݲ�����
�۸���ԭ��ع���ԭ�������ӴӸ���������������������������������������������̼������������пƬ��

����Ŀ��������Һ�ֱ���̼��������Һ��Ϻ��ܷ�����Ӧ����(����)
A.����������ҺB.����������Һ
C.����������ҺD.�Ȼ�����Һ
����Ŀ����ѧ��Ӧ���������������������������
��1��ijѧ��Ϊ��̽��п�����ᷴӦ�����е����ʱ仯������400mLϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų���������ʵ���¼���£��ۼ�ֵ����
ʱ�䣨min�� | 1 | 2 | 3 | 4 | 5 |
���������mL������״���� | 100 | 240 | 464 | 576 | 620 |
����һʱ��Σ�ָ0��1��1��2��2��3��3��4��4��5min����Ӧ�������___________��
����3��4����ʱ����������Ũ�ȱ仯����ʾ�ĸ÷�Ӧ����______________��������Һ������䣩
��2����һѧ��Ҳ��ͬ����ʵ�飬���ڷ�Ӧ̫�죬���ÿ��Ʋ�������������������������м���������������Һ�Լ�����Ӧ���ʣ�����Ϊ�����е���_________��
A������ˮ B��KCl��Һ C��KNO3��Һ D��CuSO4��Һ
��3��ij�¶�����4L�ܱ������У�3����̬����X��Y��Z�����ʵ�����ʱ��仯������ͼ��

�ٸ÷�Ӧ�Ļ�ѧ����ʽ��__________________________��
����5minʱ���÷�Ӧ�ﵽ��ƽ��״̬�����п���Ϊ�жϷ�Ӧ�Ѵﵽ��״̬����_______��
A��X��Y��Z�ķ�Ӧ�������
B������������ѹǿ���ֲ���
C��X��Y�ķ�Ӧ���ʱ�Ϊ3��1
D������1mol Y��ͬʱ����2mol Z
��2min��X��ת����Ϊ_____________________��
����Ŀ��I.ij�¶�ʱ����2L������A��B�������ʼ��ת����Ӧ��A��B���ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�����ݷ����ã���8����ʱAΪ0.2mol ��BΪ0.5mol��
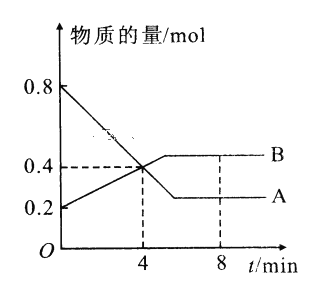
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ_________________________��
(2)��Ӧ��ʼʱ��4minʱ��A��ƽ����Ӧ����Ϊ________��
(3)4minʱ����Ӧ�Ƿ��ƽ��״̬��________(����������������)�� 8minʱ��V��________V��(����>����<����������)��
II����2L�ܱ������У�800��ʱ��Ӧ2NO(g)��O2(g) ��2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
(1)������Ӧ________(������������������)���淴Ӧ��
(2)��ͼ��ʾ����ʾNO2�仯���ߵ���________��
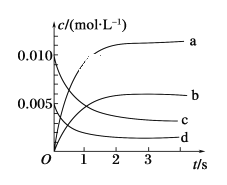
(3)��˵���÷�Ӧ�Ѵﵽƽ��״̬����________(�����)��
a. v(NO2)��2v(O2)�������� b.������ѹǿ���ֲ���
c. v��(NO)��2v��(O2) d. �������ܶȱ��ֲ���