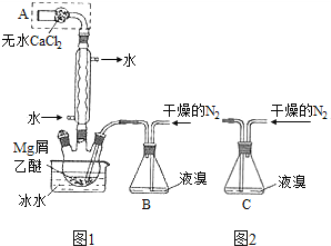
��Ŀ����
����Ŀ��
��1��ij��̬�����ﻯѧʽΪRO2���ڱ�״���£�1.28 g������������Ϊ448 mL������������Ħ������Ϊ____________��R�����ԭ������Ϊ____________��
��2����֪CO��CO2�Ļ�����������Ϊ15 g���ڱ�״�������Ϊ8.8 L�������֪�û�������к�CO________ g������CO2�ڱ�״�������Ϊ____________ L��
��3����4 g NaOH����____________gˮ�У�����ʹÿ100��ˮ����������1��Na����
��4��ͬ��ͬѹ��SO2 �� He�ܶ�֮��Ϊ ����������ȣ�������������֮��____________
���𰸡�
��1��64g��mol��1 32
��2��4��5.6��
��3��180
��4��16��1��1��16
��������
�����������1������£�1.28g������������Ϊ448mL�������ʵ���Ϊ![]() =0.02mol���ʸ��������Ħ������=
=0.02mol���ʸ��������Ħ������=![]() =64g/mol��R�����ԭ������Ϊ64-32=32���ʴ�Ϊ��64g/mol��32��
=64g/mol��R�����ԭ������Ϊ64-32=32���ʴ�Ϊ��64g/mol��32��
��2�������������Ϊ8.96L�������ʵ���Ϊ![]() =0.393mol������������CO�����ʵ���Ϊxmol��CO2�����ʵ���Ϊymol����28x+44y=15��x+y=0.393�����x=0.15mol��y=0.25mol������m(CO)=0.15mol��28g/mol=4.2g�������0.25mol������̼���Ϊ22.4L/mol��0.1mol=5.6L���ʴ�Ϊ��4.2��5.6��
=0.393mol������������CO�����ʵ���Ϊxmol��CO2�����ʵ���Ϊymol����28x+44y=15��x+y=0.393�����x=0.15mol��y=0.25mol������m(CO)=0.15mol��28g/mol=4.2g�������0.25mol������̼���Ϊ22.4L/mol��0.1mol=5.6L���ʴ�Ϊ��4.2��5.6��
��3��NaOH�����ʵ���Ϊ![]() =0.1mol����ˮ�����ʵ���Ϊx����ÿ100��H2O��������һ��Na+����
=0.1mol����ˮ�����ʵ���Ϊx����ÿ100��H2O��������һ��Na+����![]() ��
��![]() �����x=10mol����ˮ������Ϊ10mol��18g/mol=180g����4g NaOH����180��ˮ�У�����ʹÿ100��H2O��������һ��Na+��
�����x=10mol����ˮ������Ϊ10mol��18g/mol=180g����4g NaOH����180��ˮ�У�����ʹÿ100��H2O��������һ��Na+��
��4����ͬ�����£��ܶ�֮�ȵ���Ħ������֮�ȣ���SO2�뺤�����ܶ�֮��=64g/mol��4g/mol=16��1����ͬ�����£�������ͬ���������֮����Ħ�������ɷ��ȣ�����������������=4g/mol��64g/mol=1��16���ʴ�Ϊ��16��1��1��16��

 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�����Ŀ�������ʵ�������������������ʵ��Ӧ�ã������±���������һ���б����ĵ�����������գ�
ʵ�� | ���� |
(1)���������� | ���� |
(2)��������Ũ������ | |
(3)��ʳ���ؽ������ӣ����������ȴ�����ţ�������ⶾ�� | |
(4)��������Һ�м����������͵��������Һ���ֳ��� | |
(5))���þƾ���ϴ�˿� | |
(6)����ʳ�� |