��Ŀ����
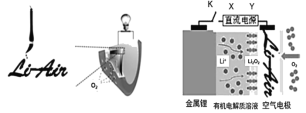
����Ŀ���������ƿ������ڸ��Ƶر�ˮ�ʣ��������ؽ���Ԫ�ط�ˮ�������ೱ��Ҳ������Ӧ�������ȡ���ҵ�������������Ƶ���Ҫ������ͼ��

��֪CaO2��8H2O�ʰ�ɫ������ˮ��������350�����ҿ�ʼ�ֽ�ų�������
(1)������������ȡCaO2��8H2O�Ļ�ѧ����ʽ��___��
(2)������ˮϴ��������Һ���Ƿ�Cl-������ȷ������___��
(3)����ʱ���ñ�ˮ�����¶���0���������������CaO2��8H2O���ʣ������ԭ����(д������)��___����___��
(4)�ⶨ��Ʒ��CaO2������ʵ�鲽�裺
��һ����ȷ��ȡag��Ʒ��������ƿ�У�������������ˮ������bgKI���壬
�ٵ�������2mol��L-1��H2SO4��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊcmol��L-1��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��Һ
VmL��(��֪��I2��2S2O32-=2I����S4O62-)
��CaO2����������Ϊ___(����ĸ��ʾ)��
��ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�CaO2��������������___(��������������ƫ��������ƫ����)��ԭ����___��
���𰸡�CaCl2+H2O2+2NH3+8H2O=CaO2��8H2O��+2NH4Cl ȡ���һ��ϴ��Һ���Թ��У��μ������ữ����������Һ�����Ƿ������ɫ���� �¶ȵͿɼ��ٹ�������ķֽ⣬��߹�������������� �÷�Ӧ�Ƿ��ȷ�Ӧ���¶ȵ����������CaO2��8H2O�IJ��� ![]() ��100% ƫ�� ����̫����S2O
��100% ƫ�� ����̫����S2O![]() �ڵζ������б���������
�ڵζ������б���������
��������
��������ͼ�ɵã��Ȼ�������ˮ��ͨ�백����30%��˫��ˮ��Ӧ��CaCl2��H2O2��2NH3��8H2O=CaO2��8H2O����2NH4Cl�����˺�õ�����ƷNH4Cl������ΪCaO2��8H2O���Գ�������ˮϴ��ȥ��������ʣ������Ҵ�ϴ�������뿾���п��ƿ�����¶ȵ���350����к�ɵõ���Ʒ�������ƣ��ݴ˷������
(1)��ʵ���Ŀ�����Ʊ�CaO2��8H2O���壬���ݷ����������еij���ӦΪCaO2��8H2O�����������غ��жϻ�Ӧ��NH4Cl���ɣ���Ӧ�Ļ�ѧ����ʽΪCaCl2��H2O2��2NH3��8H2O=CaO2��8H2O����2NH4Cl��
(2)��Һ�к��д�����Cl����Ϊ������ϴ�Ӹɾ���Ӧ���ϴ�ӣ����ݼ���Cl���ķ���������ϡ�����ữ����������Һ���顣������ˮϴ���Ƿ�ϸ�ķ�����ȡ���һ��ϴ��Һ�������Թ��У��ٵμ�ϡ�����ữ����������Һ�����Ƿ������ɫ������
(3)����ʱ���ñ�ˮ�����¶���0 �����ң������ԭ�����¶ȵͿɼ��ٹ�������ķֽ⣬��߹�������������ʣ��÷�Ӧ�Ƿ��ȷ�Ӧ���¶ȵ����������CaO2��8H2O�IJ��ʣ�
(4)�ٸ���ʵ�������CaO2��KI�����ɵⵥ�ʣ�������Ӧ�Ļ�ѧ����ʽΪ��CaO2��2KI��2H2SO4=I2��CaSO4��K2SO4��2H2O�����ݷ�Ӧ�����ӷ���ʽ��CaO2��4H����2I��=Ca2����2H2O��I2��I2��2S2O32-=2I����S4O62-���ɵù�ϵʽ��CaO2��2S2O32-����CaO2������Ϊ![]() =0.036cVg��CaO2����������Ϊ
=0.036cVg��CaO2����������Ϊ![]() ��100%��
��100%��
��S2O32-�л�ԭ�ԣ����ڵ���̫����S2O32-�ڵζ������б��������������������ƫ��CaO2�����������ı���ʽ��֪ʹ������ƫ�ߡ�