��Ŀ����
19���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ��û�ѧ������գ�| �� ���� | ��A | ��A | ��A | ��A | VA | ��A | ����A | 0�� |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� |
 ��Ԫ�آ�ԭ�Ӻ��ں���6�����ӵ�ԭ�ӿɱ�ʾΪ137N��
��Ԫ�آ�ԭ�Ӻ��ں���6�����ӵ�ԭ�ӿɱ�ʾΪ137N����2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ��HClO4��
��3��Ԫ�آ�����γɵĻ�����ĵ���ʽΪ��
 ��
����4���ݡ��ޡ�������Ԫ���γɵļ����ӣ����Ӱ뾶�ɴ�С��˳����S2-��K+��Mg2+��
��5��ʵ������Ԫ�آ۵��⻯����ȡ�۵ĵ��ʵķ�ӦΪ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
���� ��Ԫ�������ڱ��е�λ�ÿ�֪����ΪC����ΪN����ΪO����ΪNa����ΪMg����ΪS����ΪCl����ΪAr����ΪK��
��1����ΪN��ԭ�Ӻ�����2�����Ӳ㣬���������Ϊ2��5��Ԫ�آ�ԭ�Ӻ��ں���6�����ӣ�������Ϊ13��ԭ�ӷ���AZX�����½�Z���������������Ͻ�A������������X����Ԫ�ط��ţ�
��2��������������ˮ�����У�������ǿ�Ļ������Ǹ����
��3��Ԫ�آ�����γɵĻ�����ΪCS2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�
��4�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����ӵĵ��Ӳ�Խ�࣬���Ӱ뾶Խ��
��5��ʵ������Ԫ�آ۵��⻯����ȡ�۵ĵ��ʣ�Ӧ�ǹ��������ڶ�����������������������������ˮ��
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪC����ΪN����ΪO����ΪNa����ΪMg����ΪS����ΪCl����ΪAr����ΪK��
��1����ΪN��ԭ�Ӻ�����2�����Ӳ㣬���������Ϊ2��5��ԭ�ӽṹʾ��ͼΪ ��Ԫ�آ�ԭ�Ӻ��ں���6�����ӣ�������Ϊ13����ԭ�ӷ���Ϊ137N���ʴ�Ϊ��
��Ԫ�آ�ԭ�Ӻ��ں���6�����ӣ�������Ϊ13����ԭ�ӷ���Ϊ137N���ʴ�Ϊ�� ��137N��
��137N��
��2��������������ˮ�����У�������ǿ�Ļ�������HClO4���ʴ�Ϊ��HClO4��
��3��Ԫ�آ�����γɵĻ�����ΪCS2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����ӵĵ��Ӳ�Խ�࣬���Ӱ뾶Խ�����Ӱ뾶Ϊ��S2-��K+��Mg2+���ʴ�Ϊ��S2-��K+��Mg2+��
��5��ʵ������Ԫ�آ۵��⻯����ȡ�۵ĵ��ʣ�Ӧ�ǹ��������ڶ�����������������������������ˮ����Ӧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2�����ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ�ע���������뾶�Ƚϣ��ѶȲ���ּ�ڿ���ѧ���Ի����Ĺ��̣�

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д������� ���ܶ� �۷������� ��ԭ��������
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �٢� |
| A�� | Al3+��S${\;}_{4}^{2-}$�����ʵ���Ũ��֮��Ϊ2��3 | |
| B�� | SO${\;}_{4}^{2-}$�����ʵ���Ϊ0.5mol | |
| C�� | 1L��Һ�к����������ӹ�2.5NA�� | |
| D�� | ��1L 0.5mol•L-1Al2��SO4��3��Һ��ȡ��100mL��Al3+Ũ����Ϊ1mol•L-1 |
| A�� | 0.168�� | B�� | 0.14�� | C�� | 0.336�� | D�� | 0.24�� |
| A�� | 235U��239 Puԭ�Ӻ�����4������ | |
| B�� | �˷�Ӧ����ʹ�õ���ˮ��ˮ��Ϊͬλ�� | |
| C�� | ʯ�͡���Ȼ���Ȼ�ʯȼ�����ڿ�������Դ | |
| D�� | ����ʹ�ú��ܿ���Ч��������������ŷ� |
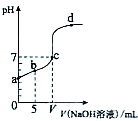 ��10.00ml 0.01mol/LijһԪ��HA��Һ����μ���0.01mol/L NaOH��Һ����ҺpH�仯������ͼ��ʾ������˵������ȷ���ǣ�������
��10.00ml 0.01mol/LijһԪ��HA��Һ����μ���0.01mol/L NaOH��Һ����ҺpH�仯������ͼ��ʾ������˵������ȷ���ǣ�������| A�� | HA������ | |
| B�� | b���ʾ����Һ�У�2c��Na+���Tc��A-��+c��HA�� | |
| C�� | b��c��d���ʾ����Һ�У�c��Na+��+c��H+���Tc��A-��+c��OH-�� | |
| D�� | c��ʱ��V=10.00 ml |
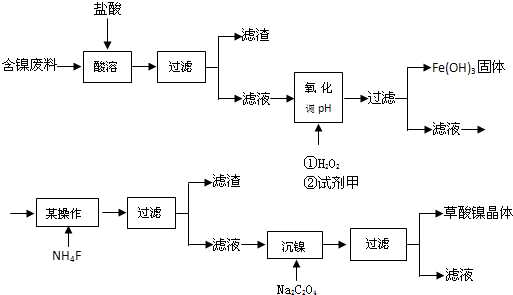
 ��д���õĵ���Ľṹ��ʽ��CH2=CH2��CH2=CH-CN��C6H5-CH=CH2
��д���õĵ���Ľṹ��ʽ��CH2=CH2��CH2=CH-CN��C6H5-CH=CH2 ��
��