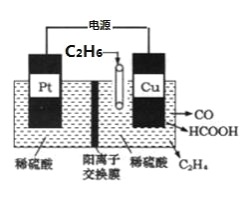
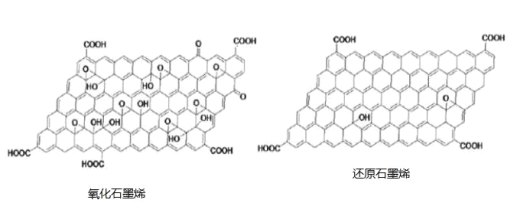
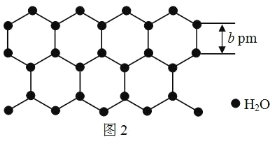
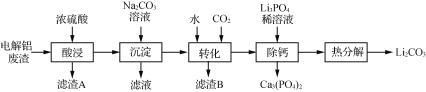
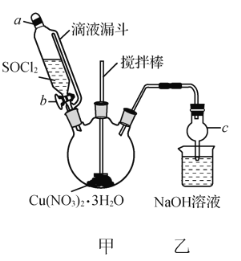
��Ŀ����
����Ŀ����1����0.1mol/LNaOH��Һ�ֱ�ζ������Ϊ20.00mL��Ũ�Ⱦ�Ϊ0.1mol/L������ʹ�����Һ���õ��ζ���������ҺpH�����NaOH��Һ������仯�������ζ����ߡ�

�ٵζ������������____������I������II������
��V1��V2�Ĺ�ϵ��V1____V2������������������������������
��M���Ӧ����Һ�У������ӵ����ʵ���Ũ���ɴ�С��˳����____��
��2��Ϊ���о������ܽ�ƽ��ͳ���ת����ijͬѧ�������ϲ��������ʵ�顣�������ϣ�AgSCN�ǰ�ɫ��������ͬ�¶��£��ܽ�ȣ�AgSCN��AgI��
�������� | ���� |
����1����2mL0.005mol/LAgNO3��Һ�м���2mL0.005mol/LKSCN��Һ�����á� | ���ְ�ɫ���� |
����2��ȡ1mL�ϲ���Һ���Թ��У��μ�1��mol/LFe(NO3)3��Һ�� | ��Һ���ɫ |
����3������2����Һ�У���������5��3mol/LAgNO3��Һ�� | ����a����Һ��ɫ��dz |
����4������1�����µ���Һ�м���5��3mol/LKI��Һ�� | ���ֻ�ɫ���� |
��д������2����Һ���ɫ�����ӷ���ʽ____��
�ڲ���3������a��____��
���û�ѧƽ��ԭ�����Ͳ���4��ʵ������____��
���𰸡�I �� c(CH3COO-)��c(Na+)��c(H+)��c(OH-) Fe3++3SCN-![]() Fe(SCN)3 ���ְ�ɫ����
Fe(SCN)3 ���ְ�ɫ���� ![]() ������KI����Ϊ�ܽ�ȣ�
������KI����Ϊ�ܽ�ȣ�![]() ��
��![]() ��
��![]() ��Ӧ����AgI��ɫ������
��Ӧ����AgI��ɫ������![]() ��AgSCN���ܽ�ƽ�������ƶ�
��AgSCN���ܽ�ƽ�������ƶ�
��������
��1�������������ᣬHCl��ǿ�ᣬŨ����ͬ�Ĵ����HCl��HCl��������Ũ�ȸ���pH��С��
�����������ֱ���NaOHǡ����ȫ��Ӧʱ���ɴ����ơ��Ȼ��ƣ��������Լ��ԡ��Ȼ��������ԣ�
����ϵ���غ��pH=7��������
��2���������Ӻ���������������Ժ�ɫ���������Ӻ������������������������
���ܽ�ȣ�AgSCN��AgI��AgSCN��AgI������ͬ���ܽ��Խ��KspԽ��Ksp��ij���ת��ΪKspС�ij�����
![]() Ũ�Ⱦ�Ϊ
Ũ�Ⱦ�Ϊ![]() ������ʹ�����Һ��pH������ĵ���1�����Ǵ���Ĵ���1�����Եζ������������I���ʴ�Ϊ��I��
������ʹ�����Һ��pH������ĵ���1�����Ǵ���Ĵ���1�����Եζ������������I���ʴ�Ϊ��I��
![]() �������������֮��ķ�Ӧ����ǡ����ȫ��Ӧ�õ��Ĵ�������ʾ���ԣ�Ҫʹ����Һ��ʾ���ԣ�
�������������֮��ķ�Ӧ����ǡ����ȫ��Ӧ�õ��Ĵ�������ʾ���ԣ�Ҫʹ����Һ��ʾ���ԣ�![]() ����Ҫ�����Թ��������������ĵ�������������������ٵ㣬�������������ǡ����ȫ��Ӧ���õ����Ȼ�����ʾ���ԣ�����
����Ҫ�����Թ��������������ĵ�������������������ٵ㣬�������������ǡ����ȫ��Ӧ���õ����Ȼ�����ʾ���ԣ�����![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() ��
��![]() ��Һ10mL����Һ���Ϊ
��Һ10mL����Һ���Ϊ![]() �Ĵ��ᷴӦ���õ����Ǵ���ʹ����ƵĻ�����ʾ���ԣ���ʱ����Ũ�ȴ�С
�Ĵ��ᷴӦ���õ����Ǵ���ʹ����ƵĻ�����ʾ���ԣ���ʱ����Ũ�ȴ�С![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() �������������軯����ʾ��ɫ�������ķ�ӦΪ��
�������������軯����ʾ��ɫ�������ķ�ӦΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() span>�����������������ӻ�������������֮�䷴Ӧ�õ�AgSCN��ɫ�������ʴ�Ϊ�����ְ�ɫ������
span>�����������������ӻ�������������֮�䷴Ӧ�õ�AgSCN��ɫ�������ʴ�Ϊ�����ְ�ɫ������
![]() ������KI����Ϊ�ܽ�ȣ�
������KI����Ϊ�ܽ�ȣ�![]() �����������Ÿ����ܵķ���ת������
�����������Ÿ����ܵķ���ת������![]() ��
��![]() ��Ӧ����AgI��ɫ������
��Ӧ����AgI��ɫ������![]() ��AgSCN���ܽ�ƽ�������ƶ����ʴ�Ϊ��
��AgSCN���ܽ�ƽ�������ƶ����ʴ�Ϊ��![]() ������KI����Ϊ�ܽ�ȣ�
������KI����Ϊ�ܽ�ȣ�![]() ��
��![]() ��
��![]() ��Ӧ����AgI��ɫ������
��Ӧ����AgI��ɫ������![]() ��AgSCN���ܽ�ƽ�������ƶ���
��AgSCN���ܽ�ƽ�������ƶ���

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�����Ŀ��Ϊ���������ЧӦ���������ص����⣬��ѧ���Dz���̽��CO2�IJ�������Դ����������������CH4����CO2��ת��ΪCO��H2���ȼ�ϵ��о��ɹ��Ѿ�������ˮ������
��֪����CH4(g)ʮH2O(g)=CO(g)+3H2(g) ��H1=+206.4kJ/mol
��CO(g)+H2O(g)=CO2(g)+H2(g) ��H2=-41.2kJ/mol
T1��ʱ����2L�����ܱ������м���2molCH4��1molCO2������ø��о��ɹ�ʵ���������£�
ʱ��/s | 0 | 10 | 20 | 30 | 40 | 50 | 60 |
CO2/mol | 1 | 0.7 | 0.6 | 0.54 | 0.5 | 0.5 | 0.5 |
H2/mol | 0 | 0.6 | 0.8 | 0.92 | 1 | 1 | 1 |
��ش��������⣺
(1)���о��ɹ����Ȼ�ѧ����ʽ��CH4(g)+CO2(g)=2CO(g)+2H2(g) ��H=__��
(2)30sʱCH4��ת����Ϊ__��20~40s��v(H2)=__��
(3)T2��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ1.5����T2__T1(����>����=������<����)
(4)T1��ʱ��Ӧ�۴ﵽƽ��ı�־Ϊ__��
A�������������ܶȲ���
B����ϵѹǿ�㶨
C��CO��H2�������������ұ��ֲ���
D��2v(CO)��=v(CH4)��
(5)������Ӧ�۴ﵽƽ��������������䣬��70sʱ�ټ���2molCH4��1molCO2���˿�ƽ����ƶ�����Ϊ__(�������ƶ���������
(6)��ͼ��֪��Ӧ��t1��t2��t3ʱ���ﵽ��ƽ�⣬����t2��t4��t8ʱ���ı������������ж�t2ʱ�ı������������__����t4ʱ�����¶ȣ�t5ʱ�ﵽƽ�⣬t6ʱ�����˷�Ӧ��Ũ�ȣ��벹t4~t6ʱ�淴Ӧ������ʱ��Ĺ�ϵ����___��