��Ŀ����
�������ӷ���ʽ��������ʵ�������ȷ����( )
| A��Ca(HCO3)2��Һ�м�������NaOH��Һ��Ca2++2HCO3-+2OH-=CaCO3��+CO32-+H2O |
| B��������Һ�м��������Ba(OH)2��Һ��Al3++2SO42-+2Ba2++4OH-=A1O2-+2BaSO4+2H2O |
| C������������Һ�м��������ˮ��Al3++4NH3��H2O=A1O2-+4NH4++2H2O |
| D����������������ϡ���Fe3O4+8H++NO3-=3Fe3++NO��+4H2O |
B
�������������A���������������Ƶ���̼�������Һ�У�̼����Ʋ���Ҫ���㶨��ɶ��ɣ�����B���������������� ��������Al3+��SO42-���㶨��ɶ��ɣ��ԣ�C����ˮ�����ܽ���������������D�������������غ㡢����غ�͵�ʧ�����غ㣬����
���㣺�������ӷ�Ӧ�����ӷ���ʽ�����ж�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�������ӷ���ʽ��ȷ����
A��̼��ƺ����ᷴӦ��CO32-��2H�� H2O��CO2�� H2O��CO2�� |
B��������������Һ�м�������Һ��Ba2����SO42- BaSO4�� BaSO4�� |
C����ϡ������Һ�м�����3Fe��6H�� 3Fe3����3H2�� 3Fe3����3H2�� |
D������������Һ�м����Ag����C1�� AgCl�� AgCl�� |
���з���ʽ��ȷ���ǣ� ��
A�����Ȱ���������ˮ��NH2Cl��H2O NH2OH��HCl NH2OH��HCl |
| B���ں���Mg2+��HCO3-������ˮ�м�������ij���ʯ��ˮ�� Ca2+ +Mg2+ +2OH- +2HCO3-=CaCO3��+MgCO3��+2H2O |
C����ʱ����к�Mg2+��HCO3-������ˮ��Mg2+ +2HCO3-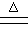 Mg(OH)2��+2CO2�� Mg(OH)2��+2CO2�� |
| D��Na2FeO4���ǻ��������ֿ�������������������������ԭ��Ϊ�� |
�������ʵ�ˮ��Һ���ܵ��磬�����ڷǵ���ʵ���
| A��SO2 | B��Cl2 | C��MgSO4 | D��KOH |
�������ӷ���ʽ��ȷ���ǣ� ��
| A��NaHSO3��Һ��Ͷ��������Ʒ�ĩ��4HSO3- +2Na2O2 = 4SO32- + O2�� + 4Na+ |
| B���Ȼ�����Һ�м��������ˮ��Al3����4NH3��H2O==AlO2-��4NH4+��2H2O |
| C������������Һ��̼����þ��Һ��Ӧ��HCO3-+Ca2++OH-��CaCO3��+H2O |
| D����1 mol/L NaAlO2��Һ��1.5 mol/L HCl��Һ�����������Ȼ�ϣ� |
���и���������ָ����Һ��һ���ܴ����������
| A���������ⸯʴ����Һ�У�NH4+��Ba2+��AlO2����Cl�� |
| B��c(H+)/ c(OH��)��1012����Һ�У�NH4+��Al3+��NO3����Cl�� |
| C����ˮ�����c(H+)��1��10��14mol��L��1����Һ�У�Ca2+��K+��Cl����HCO3�� |
| D�����ȳʺ�ɫ����Һ�У�Na+��CO32����Fe3+��Cl�� |
�������ӷ���ʽ��д��ȷ����
| A��ͭм�м��������Ȼ�����Һ��Fe3++ Cu= Fe2++ Cu2+ |
| B������ʯ�м�������Ũ���CaCO3+2H+=Ca2++CO2��+H2O |
| C��������Һ�м�����������������Һ��Ba2++OH-+H++SO42-=BaSO4��+H2O |
| D��̼�������Һ�м�����������������Һ��HCO3-+OH-=CO32-+H2O |
ʵ����������һ�ֽ����������ӣ�ˮ����������ӿɺ��ԣ��Ļ����Һ�����ڻ����Һ���������ӵ����ʵ���Ũ�Ⱦ�Ϊ1 mol/L�������ĸ�ѡ�����ܴﵽ��Ŀ�ĵ���
| A��Ca2+��K+��OH?��Cl?��NO3? |
| B��Fe2+��H+��Br?��NO3?��Cl? |
| C��Na+��K+��SO42?��NO3?��Cl? |
| D��Al3+��Na+��Cl?��SO42?��NO3? |
�����£����и���������ָ����Һ��һ���ܴ����������
| A������ҺAl2O3����Һ��Na+��K+��HCO��3��NO��3 |
| B��0.1mol��L-1Ca��ClO��2��Һ��K+��Na+��I����Cl�� |
| C����ʹ�����Ժ�ɫ����Һ��K+��Fe2+��Cl����NO��3 |
| D������KSCN�Ժ�ɫ����Һ��Na+��Mg2+��Cl����SO2��4 |