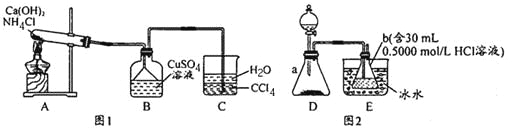
��Ŀ����
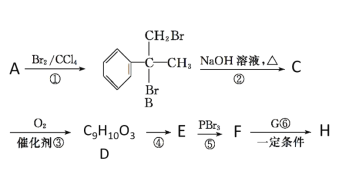
����Ŀ����ͼ��һЩ�����ĵ��ʣ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʱ���ȥ�����³�ѹ�£�AΪ��ɫ�ж����壬BΪ����ɫ��ĩ��C��EΪ�������ʣ���Ӧ![]() ��Ϊ��ҵ�ϵ���Ҫ��Ӧ��
��Ϊ��ҵ�ϵ���Ҫ��Ӧ��

��ش��������⣺
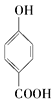
��1��![]() �Ļ�ѧʽΪ ______ ��
�Ļ�ѧʽΪ ______ ��
��2��д��C��NaOH��Һ��Ӧ����L�����ӷ���ʽ�� ______ ��
��3��д��B��C���·�Ӧ����E��F�Ļ�ѧ����ʽ�� ______ ��
��4��д��D��J��ϡ��Һ��Ӧ����G�����ӷ���ʽ�� ______ ��
���𰸡�NaHCO3 2Al��2OH-��2H2O��2AlO2-��3H2�� 2Al��Fe2O3![]() Al2O3��2Fe H2O + CO32- +CO2=2HCO3-
Al2O3��2Fe H2O + CO32- +CO2=2HCO3-
��������
BΪ����ɫ��ĩ��ӦΪFe2O3��AΪ��ɫ�ж����壬���ת����ϵ������֪A���л�ԭ�ԣ���AΪCO����DΪCO2��EΪFe��˳�ƿ�֪GΪNaHCO3��JΪNa2CO3��H��FeCl2��KΪFeCl3������C����������Ӧ����Fe��F��FΪ����������������ᷴӦ�������������Ʒ�Ӧ��FӦΪAl2O3����C��Al��IΪAlCl3��LΪNaAlO2��
(1)������������֪��GΪNaHCO3��
(2)C��NaOH��Һ��Ӧ����L����������������Һ��Ӧ����ƫ�����ƺ���������Ӧ�����ӷ���ʽΪ��2Al��2OH-��2H2O��2AlO2-��3H2����
(3)B��C���·�Ӧ����E��F�Ļ�ѧ����ʽΪ��2Al��Fe2O3![]() Al2O3��2Fe ��
Al2O3��2Fe ��
(4)D��J��ϡ��Һ��Ӧ����G������̼��̼����ϡ��Һ��Ӧ��������̼�����ƣ���Ӧ���ӷ���ʽΪ��H2O + CO32- +CO2=2HCO3-��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�