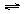��Ŀ����
25��ʱ������ƽ�ⳣ����
�ش��������⣺
��1�����ʵ���Ũ��Ϊ0.1mol/L�������������ʣ�a��Na2CO3��b��NaClO��c��CH3COONa d��NaHCO3��pH�ɴ�С��˳���ǣ�
��2��������0.1mol/L��CH3COOH��Һ��ˮϡ���̣����б���ʽ������һ����С���ǣ�
A��c��H+�� B��c��H+��/c��CH3COOH�� C��c��H+��?c��OH-�� D��c��OH-��/c��H+��
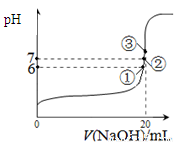
��3�����Ϊ10mLpH=2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1000mL��ϡ����pH�仯��ͼ����HX�ĵ���ƽ�ⳣ��
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH=6������Һ��
c��CH3COO-��-c��Na+��=
| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.8��10-5 | K1=4.3��10-7 K2=5.6��10-11 |
3.0��10-8 |
��1�����ʵ���Ũ��Ϊ0.1mol/L�������������ʣ�a��Na2CO3��b��NaClO��c��CH3COONa d��NaHCO3��pH�ɴ�С��˳���ǣ�
a��b��d��c
a��b��d��c
�������ţ���2��������0.1mol/L��CH3COOH��Һ��ˮϡ���̣����б���ʽ������һ����С���ǣ�
A
A
��A��c��H+�� B��c��H+��/c��CH3COOH�� C��c��H+��?c��OH-�� D��c��OH-��/c��H+��

��3�����Ϊ10mLpH=2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1000mL��ϡ����pH�仯��ͼ����HX�ĵ���ƽ�ⳣ��
����
����
������ڡ��������ڡ���С�ڡ��������ƽ�ⳣ����������HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����
HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����
��ϡ�ͺ�HX��Һ��ˮ���������c��H+������
����
������Һˮ�������c��H+��������ڡ��������ڡ���С�ڡ��������ǣ�ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С
ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С
����4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ������û��ҺpH=6������Һ��
c��CH3COO-��-c��Na+��=
9.9��10-7mol/L
9.9��10-7mol/L
������ȷ��ֵ������������1���ɵ���ƽ�ⳣ���ж����Ե�ǿ��������Խǿ�����Ӧ�ε�ˮ��̶�Խ����Һ��pH��Խ��
��2��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С��c��OH-������Kw���䣻
��3����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С��
��4��25��ʱ�����ҺpH=6��c��H+��=1.0��10-6mol/L������Kw��֪��c��OH-��=1.0��10-8mol/L���ɵ���غ��֪��c��CH3COO-��-c��Na+��=c��H+��-c��OH-����
��2��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С��c��OH-������Kw���䣻
��3����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С��
��4��25��ʱ�����ҺpH=6��c��H+��=1.0��10-6mol/L������Kw��֪��c��OH-��=1.0��10-8mol/L���ɵ���غ��֪��c��CH3COO-��-c��Na+��=c��H+��-c��OH-����
����⣺��1���ɵ���ƽ�ⳣ���ж����Ե�ǿ��������Խǿ�����Ӧ�ε�ˮ��̶�Խ����Һ��pH��Խ��
�ɱ����е����ݿ�֪������CH3COOH��H2CO3��HClO��HCO3-����ˮ��̶�Ϊa��b��d��c��pH�ɴ�С��˳����a��b��d��c���ʴ�Ϊ��a��b��d��c��
��2��A��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С����Aѡ��
B��c��H+��/c��CH3COOH��=n��H+��/n��CH3COOH������ϡ�����б�ֵ���B��ѡ��
C��ϡ���̣��ٽ����룬c��H+����С��c��OH-������c��H+��?c��OH-��=Kw��Kw���䣬��C��ѡ��
D��ϡ���̣��ٽ����룬c��H+����С��c��OH-��������c��OH-��/c��H+�����D��ѡ��
�ʴ�Ϊ��A��
��3����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С������HX��Һ��ˮ���������c��H+���ʴ�Ϊ�����ڣ�HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ�����ڣ�ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С��
��4��25��ʱ�����ҺpH=6��c��H+��=1.0��10-6mol/L������Kw��֪��c��OH-��=1.0��10-8mol/L���ɵ���غ��֪��c��CH3COO-��-c��Na+��=c��H+��-c��OH-��=9.9��10-7mol/L���ʴ�Ϊ��9.9��10-7mol/L��
�ɱ����е����ݿ�֪������CH3COOH��H2CO3��HClO��HCO3-����ˮ��̶�Ϊa��b��d��c��pH�ɴ�С��˳����a��b��d��c���ʴ�Ϊ��a��b��d��c��
��2��A��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c��H+����С����Aѡ��
B��c��H+��/c��CH3COOH��=n��H+��/n��CH3COOH������ϡ�����б�ֵ���B��ѡ��
C��ϡ���̣��ٽ����룬c��H+����С��c��OH-������c��H+��?c��OH-��=Kw��Kw���䣬��C��ѡ��
D��ϡ���̣��ٽ����룬c��H+����С��c��OH-��������c��OH-��/c��H+�����D��ѡ��
�ʴ�Ϊ��A��
��3����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С������HX��Һ��ˮ���������c��H+���ʴ�Ϊ�����ڣ�HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ�����ڣ�ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С��
��4��25��ʱ�����ҺpH=6��c��H+��=1.0��10-6mol/L������Kw��֪��c��OH-��=1.0��10-8mol/L���ɵ���غ��֪��c��CH3COO-��-c��Na+��=c��H+��-c��OH-��=9.9��10-7mol/L���ʴ�Ϊ��9.9��10-7mol/L��
���������⿼������ˮ�⼰���ԵıȽϡ�pH�����ϡ�͵ȣ�ע��ˮ�������Խ��Խˮ���ϡ����ǿ�ı仯������������ۺ��Խϴ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��2012?��ׯ��ģ��������ˮ��Һ�е���Ϊ����ѧ��ѧ����Ҫ���ݣ���֪�������ʵĵ��볣��ֵ��25�棩��
��2012?��ׯ��ģ��������ˮ��Һ�е���Ϊ����ѧ��ѧ����Ҫ���ݣ���֪�������ʵĵ��볣��ֵ��25�棩��
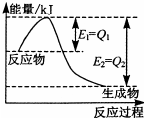 ���û�ѧ��Ӧԭ���о��������ȵȵ��ʼ��仯����ķ�Ӧ����Ҫ�����壮
���û�ѧ��Ӧԭ���о��������ȵȵ��ʼ��仯����ķ�Ӧ����Ҫ�����壮