��Ŀ����
��ʵ������,������̼�����ơ��Ȼ��ơ��Ȼ�淋������ܽ�ȵIJ��죬ͨ������ʳ��ˮ�����Ͷ�����̼��Ӧ�����̼�����ƾ��壬��Ӧԭ���������»�ѧ����ʽ��ʾ��NH3+CO2+NaCl+H2O====NH4Cl+NaHCO3(����)
���ݴ�ԭ�������Ƶ�̼�����ƾ��壬ijУѧ�����������ʵ��װ�ã�����Bװ���е��Թ��������а����Ȼ��Ƶ���Һ���Ҷ��߾��Ѵﵽ���ͣ�

(1)Aװ������������Ӧ�����ӷ���ʽΪ_____________��Cװ����ϡ���������Ϊ_________________________��
(2)�±������г�������������ڲ�ͬ�¶��µ��ܽ������(g��l00gˮ)
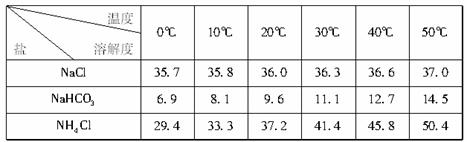
���ձ������ݣ������Bװ����ʹ�ñ�ˮ����Ϊ__________________________________��
(3)��Уѧ���ڼ�������װ�������Ժ����ʵ�飬���û�еõ�̼�����ƾ��壬ָ����ʦָ��Ӧ��_____________װ��֮��(��д��ĸ)����һ��ʢ��____________��ϴ��װ��,��������_________________________________________________________________.
(4)���øĽ����װ�ý���ʵ��,��B�е��Թ��������˾���,����Ҫ�IJ�����õ���һ�ִ����ľ��塣���������Լ������ᡢŨ��ˮ����ʯ�ҡ�����ˮ�����ñ������ṩ���Լ�(ֻ��һ��)���Թܡ��ƾ��Ƶ���Ҫ������ͨ����ʵ���жϸþ�����̼�����ƾ��壬������̼����炙�ʳ�ξ��壬��������������ʵ��������:_________________________________________��
(5)����Уѧ������ʵ��ʱ,���ñ���ʳ���к�NaCl������Ϊ5.85g,ʵ���õ������NaHCO3���������Ϊ5.04g����NaHCO3�IJ���Ϊ_____________��
(1)CaCO3+2H+====Ca2++CO2��+H2O�����մ�Bװ���е��Թ����ݳ��İ��������ٶԻ�������Ⱦ
(2)�¶�Խ�ͣ�̼�����Ƶ��ܽ��ԽС����������
(3)A��B����NaHCO3��Һ(���������𰸾��ɸ���)��ȥCO2�л��е�HCl����
(4)ȡ�������������Թ��У��ھƾ����ϼ���ʹ���ַ�Ӧ���а�ɫ����ʣ�࣬���岻��NH4HCO3�����Թ���ȴ����ʣ������м����������ᣬ����Ӧ�������������ݣ�������NaHCO3��������ʳ��(���������𰸾���)
(5)60��





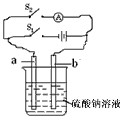
 ԭ��(ͼ��a��b���缫�IJ���ѡ�ö��̼��)��
ԭ��(ͼ��a��b���缫�IJ���ѡ�ö��̼��)��