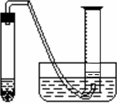
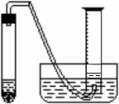
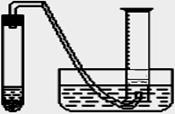
��Ŀ����
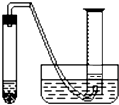 ijѧ����ͨ���ⶨ��п��������ϡ�����Ӧ����ϡ���ᷴӦ������������������ⶨ��п��п�Ĵ��ȣ����д�п��ϡ���ᡢ�Թܡ����ӡ�������Ƥ���������ܡ�������ƽ����Ͳ��ˮ�ۡ�����̨�������У���������ҩƷ��
ijѧ����ͨ���ⶨ��п��������ϡ�����Ӧ����ϡ���ᷴӦ������������������ⶨ��п��п�Ĵ��ȣ����д�п��ϡ���ᡢ�Թܡ����ӡ�������Ƥ���������ܡ�������ƽ����Ͳ��ˮ�ۡ�����̨�������У���������ҩƷ����1��ʵ��ʱ����ⶨ��п�����������������֮�⣬����Ҫ�ⶨ
��2�������Dz����ռ������������������ļ������裺
�ٵ�����Ͳ����Һ��߶�ʹ֮��ͬ��
��ʹ�Թܺ���Ͳ�ڵ����嶼��ȴ�����£�
�۶�ȡ��Ͳ������������
��������������ȷ˳���ǣ�
��3�����ʵ���в�ô�п����Ϊa g���õ������������b L��������ɱ�״������ˮ������Ӱ����Բ��ƣ���п��п�����������ļ���ʽΪ����a��b�����ػ���
��4���Ը��������������������ݵ�ʵ�鷽����
��2��Ҫȷ��������������뱣����Ͳ����������¶Ⱥ�ѹǿ��ȣ�����ڶ�ȡ��Ͳ����������֮ǰ��Ӧʹ�Թܺ���Ͳ�ڵ����嶼��ȴ�����£��ٵ�����Ͳ����Һ��߶�ʹ֮��ͬ��
��3������п��ϡ���ᷴӦ�ĵ����ϵ��֪��Ӧ��п�����ʵ���������������״�����������ʵ�����ͬ���ݴ˼���п������������
��4��������ϡ�����Ӧ�����Լ������ϡ����ȫ����Ӧ����ʣ���������Ϊ������������õ�п������������
�ʴ�Ϊ���ⶨʵ��ʱ���¶ȡ�ѹǿ��
��2����Ϊ�ų�����������Ҫ���ų���ˮ����������ģ�����Ӧǰ�����Ͳ�¶��Dz�ͬ�ģ���ˣ���Ӧ��ֹӦ����ֹͣ���ȣ�����ϵ�ָ�������ʱ����ȡ�����ܣ���һ��������ͨ����ʵ�鲻ͬ������ֹͣ����ʱ���ܵij���Ҫ������Ͳ�ڵ�Һ�棬��ʱ��ʹ��ȡ�����ܶ�ֹͣ���ȣ�Ҳ�������ˮ�ĵ�����ֹͣ���ȡ��ص����º���Ͳ�����Ե�����Ͳ�ڵ�Һ����ˮ��һ�£���ʱ��Ͳ������ѹǿҲΪ����ѹ������������ȷ�ģ�������ȷ�IJ���˳��Ϊ����ʹ���嶼��ȴ�����£��ٵ�����Ͳ����Һ��߶�ʹ֮��ͬ���۶�ȡ��Ͳ���������������ڢ٢ۣ�
�ʴ�Ϊ���ڢ٢ۣ�
��3��п��ϡ���ᷴӦ�ĵ����ϵ��֪��Ӧ��п�����ʵ���������������״�����������ʵ�����ͬ��ʵ���в�ô�п����Ϊa g���õ������������b L��������ɱ�״������ˮ������Ӱ����Բ��ƣ���п��п�����������ļ���ʽ=
| ||
| ag |
�ʴ�Ϊ��
| ||
| ag |
��4��������ϡ�����Ӧ�����Լ������ϡ����ȫ����Ӧ����ʣ���������Ϊ������������õ�п�������������;��巽��Ϊʹп������ϡ�����ַ�Ӧ���˳�ʣ����壬ϴ�ӡ����������Ӧǰ������������
�ʴ�Ϊ��ʹп������ϡ�����ַ�Ӧ���˳�ʣ����壬ϴ�ӡ����������Ӧǰ������������

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| |||||||||||||||
| |||||||||||||||||||||||||||