��Ŀ����
����Ŀ���й����ʵ����ļ�������ѧ��ѧ����Ҫ���֣���ش������й����ʵ����ļ������⡣
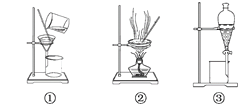
��1���ڱ�״���£�67.2 L CO2��__________mol������Ϊ_______g������__________��CO2���ӣ����к���__________mol��ԭ�ӡ�
��2���ڱ�״���£�1.7 g������ռ�����ԼΪ_________L������ͬ������_____mol H2S������ͬ����ԭ������
��3��ij��̬�����ﻯѧʽΪRO2���ڱ�״���£�1.28 g��������������448 mL�����������Ħ������Ϊ_______��R�����ԭ������Ϊ__________��
��4��ʵ���ҳ���Ũ�������������Ϊ98%���ܶ�Ϊ1.80 g��mL1�������ʵ���Ũ����_______��
��5����״���£���V L A���壨Ħ������ΪM g/mol����ȫ����0.1 Lˮ���ܶ�1 g/cm3���У�������Һ���ܶ�Ϊd g/mL�������Һ�����ʵ���Ũ��Ϊ_______mol/L��
A��![]() B��
B��![]() C��
C��![]() D��
D��![]()
���𰸡�3.0 132 3NA 6 2.24 0.15 64 g/mol 32 18.4 mol/L B
��������
��1������n=V/Vm������̼�����ʵ���������m=nM����������������N=nNA���������Ŀ����ԭ�����ʵ���Ϊ������̼��2����
��2������n=m/M���㰱�����ʵ���������V=nVm���㰱�����������Hԭ����Ŀ��ȼ�����������ʵ�����
��3������n=V/Vm����������ʵ���������M=m/n�����������Ħ����������������R�����ԭ��������
��4������c=1000��w/M�����Ũ��������ʵ���Ũ�ȣ�
��5������n=V/22.4L��mol��1��������VL����������ʵ�����������m=nM�����������������ܼ������ʵ�������Ϊ��Һ��������Ȼ������V=m/�Ѽ�����Һ��������������c=n/V�������Һ�����ʵ���Ũ�ȣ�
��1��������̼�����ʵ���Ϊ67.2L/22.4L��mol��1=3mol��
������Ϊ3mol��44g��mol��1=132g��
������̼������ĿΪ3mol��6.02��1023mol��1=1.806��1024��
��ԭ�����ʵ���Ϊ3mol��2=6mol��
��2��1.7g �������ʵ���Ϊ1.7g/17g��mol��1=0.1mol���������Ϊ0.1mol��22.4L��mol��1=2.24L��
�뺬����ͬHԭ����Ŀ����������ʵ���Ϊ0.1mol��3/2=0.15mol��
��3������������ʵ���Ϊ0.448L/22.4L��mol��1=0.02mol���������Ħ������Ϊ1.28g/0.02mol=64g��mol��1��R�����ԭ������Ϊ64-32=32��
��4���ܶ�Ϊ1.84g��cm��3����������Ϊ 98% ��Ũ���ᣬ�����ʵ���Ũ��=1000��1.84��98%/98mol��L��1=18.4 mol��L��1��
��5����״���£���������ʵ���ΪVL/22.4L��mol��1=V/22.4mol�������������Ϊ��V/22.4mol��M g��mol��1=VM/22.4g��0.1Lˮ������Ϊ��100mL��1g��mL��1=100g������Һ������Ϊ��VM/22.4g+100g�����Ը���Һ�����Ϊ��![]() L��
L��
�����Һ�����ʵ���Ũ��Ϊ��c=n/V=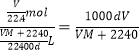 mol��L��1��
mol��L��1��
��ѡB��

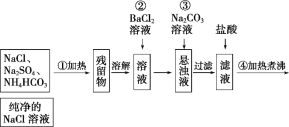
����Ŀ��ijʵ�����С��������һ�ִӷϾɵĺ�������(��Ҫ�ɷ�ΪNiO������Fe2O3��CaO��CuO��BaO�ȣ����������¹��ա�������������ͼ��
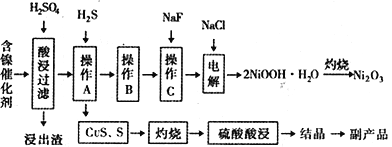
�ش�����������
��1����������Ҫ�ɷ�ΪCaSO4����2H2O��_______________�������ʡ�
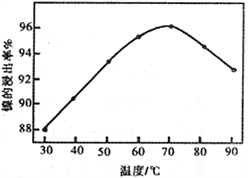
��2����ͼ��ʾ���Ľ��������¶ȵĹ�ϵ���������¶ȸ���70��ʱ�����Ľ����ʽ�������������Ni(OH)2����������ԭ����_____________________________��
��3�����������еġ�����Ʒ��Ϊ________���ѧʽ����
��4����֪�й��������↑ʼ�����ͳ�����ȫ��pH ���±���
�������� | Fe(OH)3 | Fe(OH)2 | Ni(OH)2 |
��ʼ������pH | 1.5 | 6.5 | 7.7 |
������ȫ��pH | 3.7 | 9.7 | 9.2 |
����B��Ϊ�˳�ȥ��Һ�е���Ԫ�أ�ijͬѧ���������ʵ�鷽���������A���õ���Һ�м���NaOH��Һ��������ҺpHΪ3.7��7.7�����ã����ˡ���Ը�ʵ�鷽���Ƿ���ȷ�����жϲ����������� ___________________________________����ԭ������ȷ����˵�����ɣ���ԭ������������Ը�������
��5������C��Ϊ�˳�ȥ��Һ�е�Ca2+����������Һ��F��Ũ��Ϊ3��10-3mol��L-1������Һ��![]() =________________��������ʱ��Ksp(CaF2)=2.7��10-11��
=________________��������ʱ��Ksp(CaF2)=2.7��10-11��
��6��������2NiOOH��H2O��ԭ����������
�ټ���������Cl-������������ΪClO-������1mol ClO-������OH-______________mol��
��Ni2+��ClO-��������2NiOOH��H2O��������ò���Ӧ�����ӷ���ʽΪ_________________________��
����Ŀ����������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
BԪ��ԭ�ӵĺ���p��������s��������1 |
Cԭ�ӵĵ�һ�����ĵ����ֱܷ��� I1��738 kJ/mol��I2��1 451 kJ/mol��I3��7 733 kJ/mol��I4��10 540 kJ/mol |
Dԭ�Ӻ�������p���ȫ������� |
EԪ�ص������������������IJ�Ϊ4 |
F��ǰ�������е縺����С��Ԫ�� |
G�����ڱ��ĵ����� |
��1����֪BA5Ϊ���ӻ����д�������ʽ______________________________��
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���________������ԭ�ӹ����________�Ρ�
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ![]() ��ͬѧ�����ĵ����Ų�ͼΥ����_____________________________________��
��ͬѧ�����ĵ����Ų�ͼΥ����_____________________________________��
��4��Gλ��________��________�����۵����Ų�ʽΪ________��
��5������FԪ�صķ�����________������ԭ�ӽṹ��֪ʶ���Ͳ����������ԭ����_____________________________________________________________��