��Ŀ����
��12�֣��Ȼ�����Ʒ�к�����̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ٽ�����Һ����ͼ��ʾ������в�����
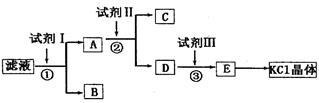
�ش��������⣺
(1)��ʼʱ��Һ��pH_____7������ڡ���С�ڡ����ڡ�������ԭ����__________________�������ӷ���ʽ��ʾ����
(2)�Լ�I�Ļ�ѧʽΪ______�����з�����Ӧ�����ӷ���ʽΪ_____________________��
(3)�Լ�II�Ļ�ѧʽΪ_____�����м����Լ�II��Ŀ����_________________________��
(4)�Լ�III��������_____�����з�����Ӧ�����ӷ���ʽΪ________________________��

�ش��������⣺
(1)��ʼʱ��Һ��pH_____7������ڡ���С�ڡ����ڡ�������ԭ����__________________�������ӷ���ʽ��ʾ����
(2)�Լ�I�Ļ�ѧʽΪ______�����з�����Ӧ�����ӷ���ʽΪ_____________________��
(3)�Լ�II�Ļ�ѧʽΪ_____�����м����Լ�II��Ŀ����_________________________��
(4)�Լ�III��������_____�����з�����Ӧ�����ӷ���ʽΪ________________________��
��1�����ڣ�1�֣� CO32-+H2O HCO3-+OH-��2�֣�
HCO3-+OH-��2�֣�
��2��BaCl2��1�֣� Ba2++SO42-=BaSO4����Ba2++CO32-=BaCO3����2�֣�
��3��K2CO3��1�֣� ��ȥ�����Ba2+��2�֣�
��4��ϡ���ᣨ1�֣� CO32-+2H+ CO2��+H2O��2�֣�
CO2��+H2O��2�֣�
 HCO3-+OH-��2�֣�
HCO3-+OH-��2�֣���2��BaCl2��1�֣� Ba2++SO42-=BaSO4����Ba2++CO32-=BaCO3����2�֣�
��3��K2CO3��1�֣� ��ȥ�����Ba2+��2�֣�
��4��ϡ���ᣨ1�֣� CO32-+2H+
 CO2��+H2O��2�֣�
CO2��+H2O��2�֣������������1����ʼ��Һ�к���̼��أ�̼�����ǿ�������Σ�̼���ˮ�⣺CO32-+H2O
 HCO3-+OH-����Һ�ʼ��ԣ�PH��7����2��Ҫ�������������������̼�����Ӧ�ȼ���������Ȼ�����Һ��̼�����������뱵���ӷ�Ӧ�������ᱵ��̼�ᱵ���������ӷ���ʽΪ��SO42-+Ba2+=BaSO4����CO32-+Ba2+=BaCO3������3��Ҫ��������ı����ӣ���������̼�����Һ��̼��غ��Ȼ�����Ӧ����̼�ᱵ���������ӷ���ʽΪCO32-+Ba2+=BaCO3������4��Ҫ���������̼�����Ҫ�μ����������ᣬ̼������Ӻ����ᷴӦ���ɶ�����̼��ˮ�����ӷ���ʽΪCO32-+2H+=CO2��+H2O��
HCO3-+OH-����Һ�ʼ��ԣ�PH��7����2��Ҫ�������������������̼�����Ӧ�ȼ���������Ȼ�����Һ��̼�����������뱵���ӷ�Ӧ�������ᱵ��̼�ᱵ���������ӷ���ʽΪ��SO42-+Ba2+=BaSO4����CO32-+Ba2+=BaCO3������3��Ҫ��������ı����ӣ���������̼�����Һ��̼��غ��Ȼ�����Ӧ����̼�ᱵ���������ӷ���ʽΪCO32-+Ba2+=BaCO3������4��Ҫ���������̼�����Ҫ�μ����������ᣬ̼������Ӻ����ᷴӦ���ɶ�����̼��ˮ�����ӷ���ʽΪCO32-+2H+=CO2��+H2O��
��ϰ��ϵ�д�
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д�
�����Ŀ
 ��CH3CH2OH��CH3CH2Br��NH4Cl��Һ������ɫҺ�壬ֻ��һ���Լ����ܰ� ���Ǽ��������Լ���(����)
��CH3CH2OH��CH3CH2Br��NH4Cl��Һ������ɫҺ�壬ֻ��һ���Լ����ܰ� ���Ǽ��������Լ���(����)
 Fe(SCN)3
Fe(SCN)3