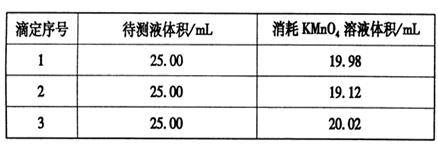
��Ŀ����
��������KOH������Һ�з���᪻���Ӧ��3 S + 6 KOH 2 K2S + K2SO3 + 3 H2O
2 K2S + K2SO3 + 3 H2O
�����������һ������K2Sx��x �� 5����K2S2O3����0.08 mol�������뺬0.06 mol KOH������Һ��ַ�Ӧǡ������a mol K2Sx��b mol K2S2O3���ټ�������KClO-KOH�Ļ����Һ����Ԫ��ȫ��ת��ΪK2SO4��ת�Ƶ���n mol����������ȷ����
 2 K2S + K2SO3 + 3 H2O
2 K2S + K2SO3 + 3 H2O�����������һ������K2Sx��x �� 5����K2S2O3����0.08 mol�������뺬0.06 mol KOH������Һ��ַ�Ӧǡ������a mol K2Sx��b mol K2S2O3���ټ�������KClO-KOH�Ļ����Һ����Ԫ��ȫ��ת��ΪK2SO4��ת�Ƶ���n mol����������ȷ����
| A��a = 2b | B��x = 2 | C��n = 0.48 | D��b = 0.02 |
AC
�������������3 S + 6 KOH
 2 K2S + K2SO3 + 3 H2O��Ӧ����0.08 mol�������뺬0.06 mol KOH������Һ��ַ�Ӧ����ʣ��0.05 mol������0.02mol K2S��0.01molK2SO3 ,ǡ������a mol K2Sx��b mol K2S2O3,�ɼ�Ԫ���غ㣺2a+2b=0.06,����Ԫ���غ㣺ax+2b=0.08���ټ�������KClO-KOH�Ļ����Һ����Ԫ��ȫ��ת��ΪK2SO4��ת�Ƶ���n mol���ɵ����غ㣺ax(6+2/x)+2b(6-2)=n�������൱��ʧ���ӵ�ȫ��0.08 mol��������n="0.08" mol*6=0.48mol������������ϵʽ��ã�x=3,a=0.02,b=0.01�ֱ��ѡ�������֤����֪AC���㡣
2 K2S + K2SO3 + 3 H2O��Ӧ����0.08 mol�������뺬0.06 mol KOH������Һ��ַ�Ӧ����ʣ��0.05 mol������0.02mol K2S��0.01molK2SO3 ,ǡ������a mol K2Sx��b mol K2S2O3,�ɼ�Ԫ���غ㣺2a+2b=0.06,����Ԫ���غ㣺ax+2b=0.08���ټ�������KClO-KOH�Ļ����Һ����Ԫ��ȫ��ת��ΪK2SO4��ת�Ƶ���n mol���ɵ����غ㣺ax(6+2/x)+2b(6-2)=n�������൱��ʧ���ӵ�ȫ��0.08 mol��������n="0.08" mol*6=0.48mol������������ϵʽ��ã�x=3,a=0.02,b=0.01�ֱ��ѡ�������֤����֪AC���㡣
��ϰ��ϵ�д�
�����Ŀ